Abstract
We recently reported that human epidermal Langerhans cells (LCs) are more efficient than dermal CD14+ DCs at priming naive CD8+ T cells into potent CTLs. We hypothesized that distinctive dendritic cell (DC) cytokine expression profiles (ie, IL-15 produced by LCs and IL-10 expressed by dermal CD14+ DCs) might explain the observed functional difference. Blocking IL-15 during CD8+ T-cell priming reduced T-cell proliferation by ∼ 50%. These IL-15–deprived CD8+ T cells did not acquire the phenotype of effector memory cells. They secreted less IL-2 and IFN-γ and expressed only low amounts of CD107a, granzymes and perforin, and reduced levels of the antiapoptotic protein Bcl-2. Confocal microscopy analysis showed that IL-15 is localized at the immunologic synapse of LCs and naive CD8+ T cells. Conversely, blocking IL-10 during cocultures of dermal CD14+ DCs and naive CD8+ T cells enhanced the generation of effector CTLs, whereas addition of IL-10 to cultures of LCs and naive CD8+ T cells inhibited their induction. TGF-β1 that is transcribed by dermal CD14+ DCs further enhanced the inhibitory effect of IL-10. Thus, the respective production of IL-15 and IL-10 explains the contrasting effects of LCs and dermal CD14+ DCs on CD8+ T-cell priming.
Introduction
Chronic diseases such as cancer and viral infections have long resisted the development of effective therapeutic vaccines. It is currently thought that such vaccines will need to effectively harness dendritic cells (DCs) to induce specific and long-lasting cellular immunity, in particular cytotoxic T cells of higher potency.1,2 Therefore, understanding the combination of signals that promote activation, proliferation, differentiation, and survival of effector CD8+ T cells is crucial for the development of such vaccines.3-5
The immunologic synapse is formed between a T cell and an antigen presenting cell (APC) and delivers a unique combination of signals that ultimately shape the type and strength of the T-cell response.6 Ag dose, costimulatory molecules, and cytokines can all direct the fate of naive T-cell differentiation to mature effector cells.7-10 IL-12 and IFN-α, as well as the costimulatory molecules CD70 and 4-1BBL, for example, regulate and fine-tune the magnitude and duration of the effector CD8+ T-cell response, as well as the nature of the elicited memory T-cell population within the immunologic synapse.11 The diverse subpopulations of DCs provide the differential composition of molecules and receptors that allows for a specialized immune response to occur.12-15 Indeed, as we have reported, Langerhans cells (LCs) are potent activators of naive CD8+ T cells compared with dermal CD14+ DCs.16 Dermal CD1a+ DCs show an intermediate activity and are able to induce effector CD8+ T-cell differentiation, although not as robustly as do LCs.16
We sought to examine the role of the epidermal and dermal DC subset-specific cytokines IL-15 and IL-10 in the differential capacity to induce CD8+ T-cell responses.16-18 In humans, IL-15–differentiated DCs are particularly efficient at priming melanoma-specific CD8+ T cells into CTLs.19,20 In contrast, IL-10–treated DCs were shown to induce anergic CD8+ T-cell responses.21,22 Given their unique cytokine expression profile and their differential ability to prime effector CD8+ T cells, we assessed the direct contribution of skin DC-specific cytokines to the quality of a primary CD8+ T-cell response.
Methods
DC subsets
In vitro DC subsets were differentiated from CD34+ hematopoietic progenitor cells that were isolated from the blood of G-CSF–mobilized healthy volunteers. Hematopoietic progenitor cells were cultured at 0.5 × 106 cells/mL in Yssel medium (Irvine Scientific) supplemented with 5% autologous serum, 50μM β-mercaptoethanol, 1% l-glutamine, 1% penicillin/streptomycin, GM-CSF (50 ng/mL; Genzyme), Flt3-L (100 ng/mL; R&D Systems), and TNF-α (10 ng/mL; R&D Systems) for 9 days. Media and cytokines were refreshed at day 5 of culture. Subsets of DCs, CD1a+CD14− (in vitro LCs) and CD1a−CD14+ DCs (in vitro CD14+ DCs) were then purified by cell sorting after staining with anti-CD1a FITC (Dako) and anti-CD14 APC mAbs (Invitrogen), yielding a purity of 95%-99%.
Epidermal LCs, dermal CD1a+ DCs, and dermal CD14+ DCs were purified from normal human skin specimens. Specimens were incubated in bacterial protease dispase type 2 for 18 hours at 4°C, and then for 2 hours at 37°C. Epidermal and dermal sheets were then separated, cut into small pieces (∼ 1-10 mm) and placed in RPMI 1640 supplemented with 10% FBS. Pooled human serum or serum-free media were used as indicated. After 2 days, the cells that migrated into the medium were collected and further enriched with a Ficoll-diatrizoate in a density of 1.077 g/dL. DC subsets were purified by cell sorting. Intracellular IL-15 expression was detected with anti–IL-15 (clone 34559; R&D Systems). All protocols were reviewed and approved by the Baylor Institute of Immunology Research Institutional Review Board.
DC–T-cell cocultures
Naive CD8+ T cells were sorted from PBMCs of healthy volunteers as CD45RA+CCR7+HLA-DR−CD8+ cells. Alternatively, cells were positively selected with the naive CD8+ T-cell isolation kit (Miltenyi Biotec). For autologous response, naive CD8+ T cells (1 × 106 cells/well) were stimulated with autologous in vitro DC subsets (5 × 104 cells/well) that were preloaded for 3 hours with the HLA-A*0201–restricted MART-1 peptide [MART-1(26-35) ELAGIGILTV] and cultured for 9-10 days in 24-well plates with 100 ng/mL CD40L (R&D Systems) and 10 U/mL IL-7 (R&D Systems). IL-2 was added at 10 U/mL at day 3. Expansion of peptide-specific CD8+ T cells was determined by counting the number of cells binding peptide MART-1–HLA-A*0201 tetramers (Beckman Coulter). Cytotoxic capacity and intracellular effector molecule expression were assessed after 2 consecutive stimulations by standard 51Cr-release assay as previously described16 and multicolor flow cytometry, respectively. A neutralizing anti–IL-15 mAb, generated in house and specific for the anti–IL-15/IL-15R complex (Clone 31C12; BIIR), and a neutralizing anti–IL-10 (Clone AB39_46.5C8.2H9; BIIR; see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article, for details on mAbs activity) were added at 5 μg/mL as indicated. A combination of anti–IL-15 (Clone MAB 647; R&D Systems) together with anti–IL-15Rα (Clone AF247; R&D Systems) was also used as indicated. For allogeneic primary responses, 1 × 105 CFSE-labeled naive T cells were cultured with DC subsets either generated in vitro or isolated from skin (DC/T-cell ratio, 1:40 unless otherwise indicated) for 6-7 days. IL-15, IL-10, TGF-β1, or a neutralizing mAb to IL-15 or IL-10 were added to the cocultures when specified. Proliferating CD8+ T cells (CFSElo) were characterized for surface expression of CD25, CD28, CCR7, and CD45RA or intracellular expression of cytotoxic effector molecules granzyme A (BD PharMingen), granzyme B (Invitrogen), and perforin (Fitzgerald). CD107a mobilization and intracellular expression of Bcl-2, IL-2, and IFN-γ accumulation were assessed after 6 hours of stimulation with autologous LCs and 0.25 μg/mL of anti-CD28 and CD49d mAbs. IL-15–neutralization assays were performed in the absence of the other common γ-chain cytokines. Biologically active trimeric CD40L (100 ng/mL; R&D Systems; aa 108-261 was used to activate DCs in all the experiments.
Synapse formation and immunolabeling
LCs or dermal CD1a+ DCs were activated with CD40L and incubated with allogeneic naive CD8+ T cells at a ratio of 1:10 for 18 hours on Poly-D-Lysine chamber slides (BD Bioscience). Cells were fixed and immune-labeled with IL-15–Alexa Fluor 488 (Clone 34559; R&D Systems), HLA-DR–Alexa Fluor 568 (G46-6; BD Biosciences), and CD8–Alexa Fluor 647 (RPA-T8; BD Biosciences). Cells were then mounted with Prolong Gold and DAPI. Confocal images were acquired with the Leica SP5 confocal with the Planapo 63×/1.4 oil objective with zoom factor 3. The contrast and brightness were adjusted with Photoshop software (Adobe Systems) to balance the 3 pseudo-colored images.
Results
IL-15 promotes priming of effector CD8+ T cells
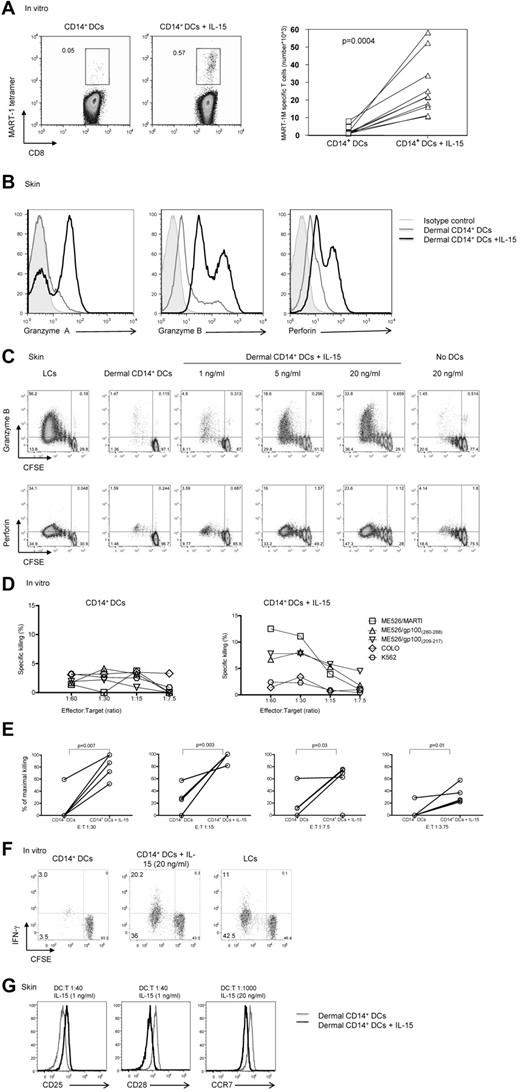
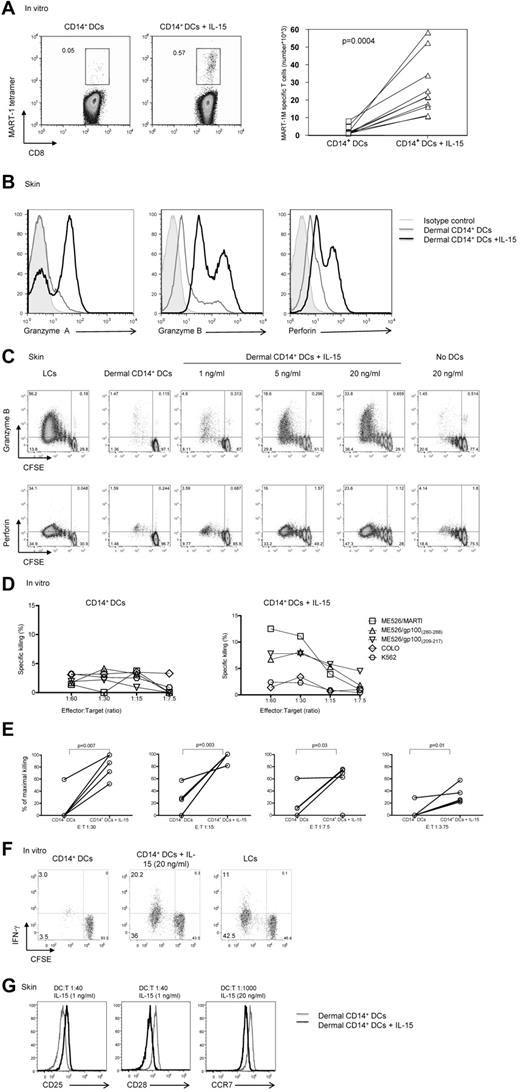
The addition of IL-15 to cocultures of HLA-A*0201+ naive CD8+ T cells and autologous MART-1 peptide–loaded in vitro CD14+ DCs (Figure 1A) enhanced the frequency (left panel) and absolute numbers (right panel) of MART-1–specific CD8+ T cells (0.57% vs 0.05%; P = .004). These cells showed enhanced expression of granzyme A, granzyme B, and perforin in a dose-dependent manner (Figure 1B black histogram vs gray and filled gray histograms, C). The expression of induced markers depended on the presence of DCs (Figure 1C). Similar results were obtained when CD8+ T cells were cocultured with DC subsets that had been generated in vitro rather than isolated from skin (not shown). CD8+ T cells cultured with CD14+ DCs in the presence of IL-15 were able to kill target cells (Figure 1D-E). In these experiments, naive CD8+ T cells were exposed twice to autologous HLA-A*0201+ in vitro CD14+ DCs that had been loaded with 3 peptides from MART-1 and gp100 proteins, with or without IL-15.
IL-15 enhances CD8+ T-cell priming. (A left) In vitro CD40L-activated HLA-A*0201+ CD14+ DCs were loaded with MART-1(26-35) peptide (3μM) and cultured with autologous naive CD8+ T cells for 9 days with or without IL-15 (20 ng/mL). CD40L and IL-7 were added on day 0, and IL-2 was added on day 3. The expansion of Ag-specific CD8+ T cells was assessed by flow cytometry with the use of MART-1(26-35)–HLA-A*0201 tetramer. Data are representative of 8 independent experiments. (A right) Graph shows the absolute MART-1(26-35)–specific cells per milliliter of 8 independent experiments. Statistical analysis was performed using paired Student t test. (B) Allogeneic naive CD8+ T cells were primed by CD40L-activated dermal CD14+ DCs with or without IL-15 for 7 days and analyzed by flow cytometry for the intracellular expression of the effector molecules: granzyme A, granzyme B, and perforin. Gray histogram shows staining with an isotype control. Data are representative of 3 independent experiments. (C) Intracellular expression of the effector molecules, granzyme B and perforin, by allogeneic CD8+ T cells primed for 7 days by CD40L-activated skin LCs or dermal CD14+ DCs without or with increasing dose of IL-15 (1, 5, and 20 ng/mL, left panel). (Right panel) Expression level granzyme B and perforin cultured for 7 days with IL-15 (20 ng/mL) and no DCs. Data are representative of 3 independent experiments. (D) The cytotoxic activity of MART-1(26-35)–specific CD8+ T cells primed by in vitro peptide–loaded autologous CD14+ DCs for 2 consecutive stimulations with or without IL-15 as assessed in a standard 51Cr release assay against an HLA-A*0201+ melanoma cell line MEL526 expressing HLA-A*0201–MART-1 complexes at indicated E:T ratios. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3 of the primary coculture. Data are expressed as mean of triplicate measurements for a single donor representative of 3 independent experiments with the use of different donors. (E) The cytotoxic activity of MART-1(26-35)–specific or gp100(209-217)–specific CD8+ T cells primed by in vitro peptide–loaded autologous CD14+ DCs for 2 consecutive stimulations with or without IL-15 as assessed in a standard 51Cr release assay against target cells expressing peptide HLA-A*0201 complexes at indicated E:T ratios. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3 of the primary coculture. Graph shows percentage of maximal killing of 5 experiments with the use of different target cells and different donors. (F) Allogeneic CD8+ T cells were primed for 8 days with sorted in vitro CD40L-activated DC subsets. Plots show the level of CFSE dilution and IFN-γ expression after 6 hours of re-stimulation with fresh DCs in the presence of monensin and anti-CD28/49d. Data are representative of 3 independent experiments. (G) Histogram shows CD25, CD28, and CCR7 receptor expression level by the viable CFSEloCD8+ T cells that were exposed to allogeneic CD40L-activated dermal CD14+ DCs with (black) or without (gray) soluble IL-15, at indicated DC/T-cell ratio and cytokine concentrations. Data are representative of 3 independent experiments.
IL-15 enhances CD8+ T-cell priming. (A left) In vitro CD40L-activated HLA-A*0201+ CD14+ DCs were loaded with MART-1(26-35) peptide (3μM) and cultured with autologous naive CD8+ T cells for 9 days with or without IL-15 (20 ng/mL). CD40L and IL-7 were added on day 0, and IL-2 was added on day 3. The expansion of Ag-specific CD8+ T cells was assessed by flow cytometry with the use of MART-1(26-35)–HLA-A*0201 tetramer. Data are representative of 8 independent experiments. (A right) Graph shows the absolute MART-1(26-35)–specific cells per milliliter of 8 independent experiments. Statistical analysis was performed using paired Student t test. (B) Allogeneic naive CD8+ T cells were primed by CD40L-activated dermal CD14+ DCs with or without IL-15 for 7 days and analyzed by flow cytometry for the intracellular expression of the effector molecules: granzyme A, granzyme B, and perforin. Gray histogram shows staining with an isotype control. Data are representative of 3 independent experiments. (C) Intracellular expression of the effector molecules, granzyme B and perforin, by allogeneic CD8+ T cells primed for 7 days by CD40L-activated skin LCs or dermal CD14+ DCs without or with increasing dose of IL-15 (1, 5, and 20 ng/mL, left panel). (Right panel) Expression level granzyme B and perforin cultured for 7 days with IL-15 (20 ng/mL) and no DCs. Data are representative of 3 independent experiments. (D) The cytotoxic activity of MART-1(26-35)–specific CD8+ T cells primed by in vitro peptide–loaded autologous CD14+ DCs for 2 consecutive stimulations with or without IL-15 as assessed in a standard 51Cr release assay against an HLA-A*0201+ melanoma cell line MEL526 expressing HLA-A*0201–MART-1 complexes at indicated E:T ratios. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3 of the primary coculture. Data are expressed as mean of triplicate measurements for a single donor representative of 3 independent experiments with the use of different donors. (E) The cytotoxic activity of MART-1(26-35)–specific or gp100(209-217)–specific CD8+ T cells primed by in vitro peptide–loaded autologous CD14+ DCs for 2 consecutive stimulations with or without IL-15 as assessed in a standard 51Cr release assay against target cells expressing peptide HLA-A*0201 complexes at indicated E:T ratios. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3 of the primary coculture. Graph shows percentage of maximal killing of 5 experiments with the use of different target cells and different donors. (F) Allogeneic CD8+ T cells were primed for 8 days with sorted in vitro CD40L-activated DC subsets. Plots show the level of CFSE dilution and IFN-γ expression after 6 hours of re-stimulation with fresh DCs in the presence of monensin and anti-CD28/49d. Data are representative of 3 independent experiments. (G) Histogram shows CD25, CD28, and CCR7 receptor expression level by the viable CFSEloCD8+ T cells that were exposed to allogeneic CD40L-activated dermal CD14+ DCs with (black) or without (gray) soluble IL-15, at indicated DC/T-cell ratio and cytokine concentrations. Data are representative of 3 independent experiments.
Cultured T cells were then tested for their ability to kill the HLA-A*0201+ melanoma cell line Mel 526 loaded with the appropriate peptides, using the HLA-A*0201− Colo cell line and MHC-class I-negative K562 as controls. As described earlier,16 CD8+ T cells primed with in vitro peptide-loaded CD14+ DCs did not kill tumor cells (Figure 1D left panel, E). In contrast, CD8+ T cells cocultured with CD14+ DCs and IL-15 yielded T cells able to kill target cells (Figure 1D right panel, E). CD8+ T cells primed with in vitro CD14+ DCs pre-exposed to IL-15 showed increased proliferation and IFN-γ production compared with unexposed CD14+ DCs (Figure 1F). The potency of CD14+ DCs exposed to IL-15 was comparable with that observed with in vitro LCs (Figure 1F). The effect of IL-15 was observed even under limiting culture conditions. Indeed, as shown in Figure 1G, low amounts of IL-15 (1 ng/mL) added to cocultures of dermal CD14+ DCs and naive CD8+ T cells or low DC/T-cell ratio of 1:1000 were sufficient to induce a notable change in the phenotype of the primed T cells such as increased expression of CD25 and decreased expression of CD28 and CCR7, respectively. Taken together, our data indicate that IL-15 potently enhances the expansion, functional avidity, and effector function of naive CD8+ T cells primed with CD14+ DCs.
DC-derived IL-15 is critical for priming effector CD8+ T cells
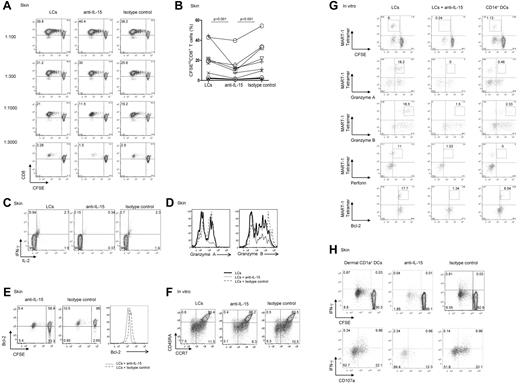
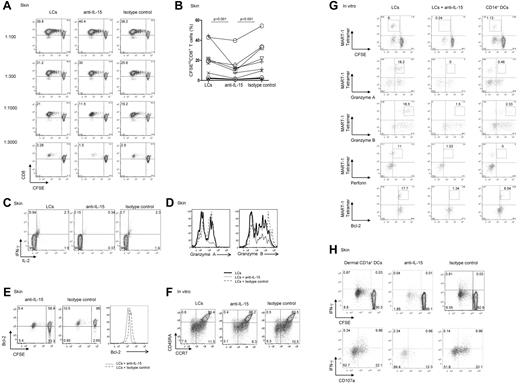
LCs but not dermal CD14+ DCs express IL-15. Thus, to establish a role for IL-15 in DC-dependent T-cell activation, cocultures of DC subsets and allogeneic naive CD8+ T cells were supplemented with either a neutralizing anti–IL-15 mAb or an isotype-matched control. As shown in Figure 2A, CD8+ T cells primed with LCs robustly proliferated, whereas dermal CD14+ DCs barely activated allogeneic CD8+ T cells.16 At high LC/T-cell ratios (1:100 or 1:300), the neutralizing anti–IL-15 did not inhibit the proliferation of CD8+ T cells, although this Ab was a potent inhibitor of T-cell proliferation induced by exogenous IL-15 (not shown). However, under limiting conditions (LC/T-cell ratio ≤ 1:1000), the anti–IL-15 mAb or a combination of anti–IL-15 and anti–IL-15Rα inhibited the proliferative response of naive CD8+ T cells by ∼ 50% (Figure 2A-B). CD8+ T cells that were cocultured with LCs and anti–IL-15 produced less IL-2 and IFN-γ (Figure 2C) and expressed lower levels of the effector proteins granzyme A and granzyme B (Figure 2D), as well as of the antiapoptotic protein Bcl-2 (Figure 2E). Costaining of CCR7 and CD45R showed that IL-15 is essential for the differentiation of effector (CCR7−CD45RA−) and central (CCR7+CD45RA−) memory CD8+ T-cell populations. Thus, CD8+ T cells cultured with autologous MART-1 peptide–loaded in vitro LCs and anti–IL-15 displayed a lower frequency of effector (3.1% vs 6.5% vs 7.5%) and to a lesser extent central memory (8.3% vs 10.5% vs 11.5%) CD8+ T-cell populations than control cultures incubated with a control Ab (Figure 2F).
Blocking DC-derived IL-15 inhibits priming of effector CD8+ T cells. (A) CD8+ T cells were labeled with CFSE and primed for 7 days with decreasing numbers of allogeneic CD40L-activated skin LCs. Neutralizing IL-15 mAb (clone MAB 647; R&D Systems) and anti–IL-15Rα (clone AF247; R&D Systems) or isotype-matched control mAbs were used as indicated. Dot plots showing the proportion of cells that diluted CFSE (CFSElo) were assessed by flow cytometry. Data are shown from 1 of 11 independent experiments. (B) Graph shows the proportions of CD8+ T cells that were primed by allogeneic CD40L-activated LCs at a DC/T-cell ratio of 1:1000 or lower, in the presence of neutralizing mAb to IL-15 or matched isotype control. Graph shows data of 11 independent experiments; P < .001. (C) Purified naive CD8+ T cells were primed for 7 days by allogeneic CD40L-activated skin LCs. Neutralizing anti–IL-15 mAb or a control mAb was added as indicated. The cultured cells were activated for 24 hours with fresh LCs. Monensin was added during the last 6 hours, and intracellular IFN-γ and IL-2 production were measured by flow cytometry. Data are representative of 3 independent experiments. (D) CFSE-labeled naive CD8+ T cells were primed for 7 days by allogeneic CD40L-activated skin LCs in the presence of neutralizing anti–IL-15 mAb or an isotype-matched control. Histograms show intracellular expression of the effector molecules granzyme A and granzyme B by the viable CD3+CD8+CFSElo T cells in response to o/n restimulation with fresh LCs as analyzed by flow cytometry. Data are representative of 3 independent experiments. (E) CD40L-activated skin LCs were used to prime allogeneic naive CFSE-labeled T cells. Neutralizing IL-15 or an isotype-matched control mAb was added to the coculture. After 7 days, the dilution of CFSE and intracellular Bcl-2 expression by the cultured CD8+ T cells were assessed by flow cytometry. Histogram shows the expression of Bcl-2 by the viable CD3+CD8+CFSElo T cells. Data are representative of 3 independent experiments. (F) CD8+ T cells were cultured with autologous peptide-loaded sorted in vitro HLA-A*0201+ LCs with a neutralizing anti–IL-15 mAb or an isotype-matched control. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3. After 10 days, cells were restimulated for 24 hours with fresh DCs, and effector memory populations were analyzed by flow cytometry that was based on the costaining with CCR7 and CD45RA. Data are representative of 3 independent experiments. (G) In vitro HLA-A*0201+ DC subsets were used to prime autologous naive CD8+ T cells. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3 of the primary coculture. Neutralizing mAb to IL-15 was added to the coculture of naive CD8+ T cells and in vitro LCs. Cells were analyzed after 2 consecutive stimulations by flow cytometry for the dilution of CFSE dye, frequency of MART-1 tetramer–binding cells, and intracellular expression of granzymes, perforin, and Bcl-2 proteins. Data are representative of ≥ 3 independent experiments. (H) CFSE-labeled naive CD8+ T cells were primed with allogeneic CD40L-activated dermal CD1a+ DCs at the indicated 1:40 DC/T-cell ratio and with anti–IL-15 or an isotype-matched control mAb. After 9 days, cells were harvested and restimulated for 6 hours with fresh autologous LCs in the presence of monensin and anti-CD28/CD49d to assess IFN-γ production and CD107a surface mobilization. (Bottom panel) Cells are gated on viable CD3+CD8+CFSElo T cells. Data are representative of 4 independent experiments.
Blocking DC-derived IL-15 inhibits priming of effector CD8+ T cells. (A) CD8+ T cells were labeled with CFSE and primed for 7 days with decreasing numbers of allogeneic CD40L-activated skin LCs. Neutralizing IL-15 mAb (clone MAB 647; R&D Systems) and anti–IL-15Rα (clone AF247; R&D Systems) or isotype-matched control mAbs were used as indicated. Dot plots showing the proportion of cells that diluted CFSE (CFSElo) were assessed by flow cytometry. Data are shown from 1 of 11 independent experiments. (B) Graph shows the proportions of CD8+ T cells that were primed by allogeneic CD40L-activated LCs at a DC/T-cell ratio of 1:1000 or lower, in the presence of neutralizing mAb to IL-15 or matched isotype control. Graph shows data of 11 independent experiments; P < .001. (C) Purified naive CD8+ T cells were primed for 7 days by allogeneic CD40L-activated skin LCs. Neutralizing anti–IL-15 mAb or a control mAb was added as indicated. The cultured cells were activated for 24 hours with fresh LCs. Monensin was added during the last 6 hours, and intracellular IFN-γ and IL-2 production were measured by flow cytometry. Data are representative of 3 independent experiments. (D) CFSE-labeled naive CD8+ T cells were primed for 7 days by allogeneic CD40L-activated skin LCs in the presence of neutralizing anti–IL-15 mAb or an isotype-matched control. Histograms show intracellular expression of the effector molecules granzyme A and granzyme B by the viable CD3+CD8+CFSElo T cells in response to o/n restimulation with fresh LCs as analyzed by flow cytometry. Data are representative of 3 independent experiments. (E) CD40L-activated skin LCs were used to prime allogeneic naive CFSE-labeled T cells. Neutralizing IL-15 or an isotype-matched control mAb was added to the coculture. After 7 days, the dilution of CFSE and intracellular Bcl-2 expression by the cultured CD8+ T cells were assessed by flow cytometry. Histogram shows the expression of Bcl-2 by the viable CD3+CD8+CFSElo T cells. Data are representative of 3 independent experiments. (F) CD8+ T cells were cultured with autologous peptide-loaded sorted in vitro HLA-A*0201+ LCs with a neutralizing anti–IL-15 mAb or an isotype-matched control. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3. After 10 days, cells were restimulated for 24 hours with fresh DCs, and effector memory populations were analyzed by flow cytometry that was based on the costaining with CCR7 and CD45RA. Data are representative of 3 independent experiments. (G) In vitro HLA-A*0201+ DC subsets were used to prime autologous naive CD8+ T cells. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3 of the primary coculture. Neutralizing mAb to IL-15 was added to the coculture of naive CD8+ T cells and in vitro LCs. Cells were analyzed after 2 consecutive stimulations by flow cytometry for the dilution of CFSE dye, frequency of MART-1 tetramer–binding cells, and intracellular expression of granzymes, perforin, and Bcl-2 proteins. Data are representative of ≥ 3 independent experiments. (H) CFSE-labeled naive CD8+ T cells were primed with allogeneic CD40L-activated dermal CD1a+ DCs at the indicated 1:40 DC/T-cell ratio and with anti–IL-15 or an isotype-matched control mAb. After 9 days, cells were harvested and restimulated for 6 hours with fresh autologous LCs in the presence of monensin and anti-CD28/CD49d to assess IFN-γ production and CD107a surface mobilization. (Bottom panel) Cells are gated on viable CD3+CD8+CFSElo T cells. Data are representative of 4 independent experiments.
We further analyzed how the LC-derived IL-15 might alter peptide-specific responses to HLA-A*0201+ in vitro LCs with the use of specific tetramers and flow cytometry (Figure 2G). The addition of anti-IL15 mAb to a coculture of in vitro MART-1 peptide–loaded LCs and autologous naive CFSE-labeled CD8+ T cells inhibited the priming of CFSElo MART-1–specific effector CD8+ T cells (0.24% vs 6%) to a level that was comparable with that induced by in vitro CD14+ DCs (Figure 2G top row). The expression of the effector molecules granzyme A (0% vs 18.2%), granzyme B (1.5% vs 18.5%), and perforin (1.03% vs 11%) followed the same trend (Figure 2G). The CD8+ T cells cultured with in vitro LCs and the anti–IL-15 Ab displayed low levels of the effector molecules granzyme A, granzyme B, and perforin (Figure 2G), a phenotype similar to that of naive CD8+ T cells cultured with in vitro CD14+ DCs. Furthermore, IL-15 was essential for maintaining Bcl-2 expression by the Ag-specific CD8+ T cells. As shown in Figure 2G, Ag-specific CD8+ T cells cultured with peptide-loaded autologous LCs and anti–IL-15 showed reduced expression of Bcl-2 (1.34% vs 17.7%). IL-15 was also essential for the priming of effector CD8+ T cells by dermal CD1a+ DCs (a DC population sharing properties of both dermal CD14+ DCs and LCs; Figure 2H) because blocking IL-15 during cocultures of dermal CD1a+ DCs and allogeneic naive CD8+ T cells inhibited IFN-γ production (Figure 2H top panel). The levels of IFN-γ expression by CFSEloCD8+ T cells correlated with the intensity of CD107a that was mobilized to the cell surface, a marker associated with T-cell cytotoxic capacity (Figure 2H bottom panel). Taken together, these data indicate that DCs activate effector CD8+ T cells through the production of IL-15.
CD14+ DC-derived IL-10 and TGF-β1 inhibit the differentiation of CD8+ T cells
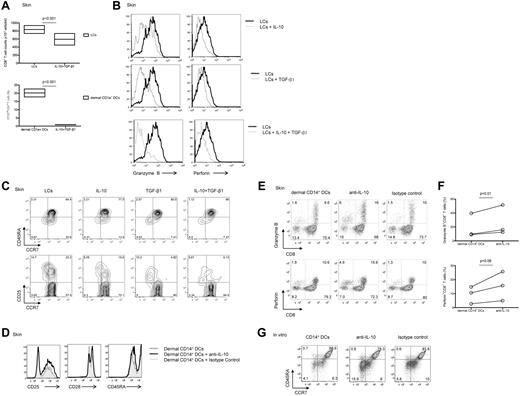
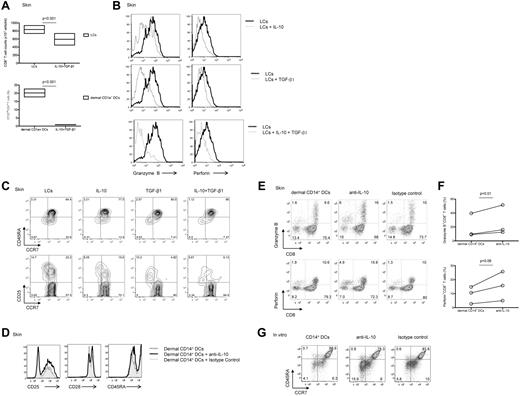
Because CD14+ DCs secrete the anti-inflammatory cytokine IL-10 and transcribe TGF-β1,16 we wondered whether these cytokines might explain the low potency of CD14+ DCs to induce high avidity CTLs. Indeed, the addition of IL-10 and TGF-β1 reduced the proliferation of CD8+ T cells induced by allogeneic skin LCs (Figure 3A). The combination of IL-10 and TGF-β1 resulted in a greater inhibition of CD8+ T-cell proliferation compared with each cytokine alone (supplemental Figure 2). Addition of either IL-10 or TGF-β1 to cocultures of naive CD8+ T cells and skin LCs yielded CFSEloCD8+ T cells that expressed low levels of granzyme B and perforin (Figure 3B top and middle rows, respectively). The combination of IL-10 and TGF-β1 resulted in a notable decreased expression of these 2 effector molecules (Figure 3B bottom row). Furthermore, addition of IL-10, TGF-β1, or the combination of the 2 cytokines to skin LCs prevented the differentiation and the activation of effector (CCR7−CD45RA−; 8.05%, 10.2%, and 5.67% vs 14.7%) and central (CCR7+CD45RA−; 15.5%, 4.82%, and 6.13% vs 22.2%) memory CD8+ T-cell populations (Figure 3C).
Dermal CD14+ DC-derived IL-10 and TGF-β1 prevent the generation of effector CTLs. (A) CFSE-labeled naive CD8+ T cells were stimulated with allogeneic CD40L-activated skin LCs (top panel) or dermal CD1a+ DCs (bottom panel) in the presence of IL-10 (10 ng/mL) and TGF-β1 (5 ng/mL). Graph shows the absolute CD8+ T-cell number or the proportion of CD8+ T cells that diluted CFSE dye (CFSElo) as measured after 7 days by flow cytometry. Boxes represent average results of 4 independent experiments; P < .001. (B) Flow cytometric analysis of granzyme B and perforin expression by the CD8+CFSElo T cells cultured for 7 days over allogeneic CD40L-activated skin LCs with (gray histogram) or without (black histogram) IL-10 and/or TGF-β1 as indicated. Results are representative of 3 independent experiments. (C) Flow cytometric analysis of CD8+ T-cell subsets (as indicated by the expression of CCR7 and CD45RA; top panel) and the activation marker CD25 (bottom panel) by CD8+ T cells cultured for 7 days over allogeneic CD40L-activated skin LCs conditioned with IL-10, TGF-β1, or a combination of the 2 cytokines. Data are representative of 3 independent experiments. (D) Flow cytometric analysis of surface receptors (CD25, CD28, and CD45RA) expression by CFSEloCD8+ T cells cultured for 6 days with allogeneic CD40L-activated dermal CD14+ DCs with a neutralizing IL-10 mAb or an isotype-matched control after reactivation with fresh autologous DCs for 24 hours before the analysis. Data are representative of 3 independent experiments. (E) Similar experiment as in panel D. Dot plots show the expression of granzyme B (top panel) and perforin (bottom panel) by the cultured viable CD3+CD8+ T cells. Data are representative of 3 independent experiments. (F) Granzyme B (top panel) and perforin (bottom panel) expression as measured by the cultured viable CD3+CD8+ T cells in 3 independent experiments; P = .01 and P = .06, respectively. (G) CD8+ T cells were cultured with autologous peptide-loaded in vitro HLA-A*0201+ CD14+ DCs in the presence of neutralizing anti–IL-10 mAb or an isotype-matched control for 10 days. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3. Cells were restimulated for 24 hours with fresh DCs, and effector memory populations were analyzed by flow cytometry that was based on the costaining with CCR7 and CD45RA. Data are representative of 3 independent experiments.
Dermal CD14+ DC-derived IL-10 and TGF-β1 prevent the generation of effector CTLs. (A) CFSE-labeled naive CD8+ T cells were stimulated with allogeneic CD40L-activated skin LCs (top panel) or dermal CD1a+ DCs (bottom panel) in the presence of IL-10 (10 ng/mL) and TGF-β1 (5 ng/mL). Graph shows the absolute CD8+ T-cell number or the proportion of CD8+ T cells that diluted CFSE dye (CFSElo) as measured after 7 days by flow cytometry. Boxes represent average results of 4 independent experiments; P < .001. (B) Flow cytometric analysis of granzyme B and perforin expression by the CD8+CFSElo T cells cultured for 7 days over allogeneic CD40L-activated skin LCs with (gray histogram) or without (black histogram) IL-10 and/or TGF-β1 as indicated. Results are representative of 3 independent experiments. (C) Flow cytometric analysis of CD8+ T-cell subsets (as indicated by the expression of CCR7 and CD45RA; top panel) and the activation marker CD25 (bottom panel) by CD8+ T cells cultured for 7 days over allogeneic CD40L-activated skin LCs conditioned with IL-10, TGF-β1, or a combination of the 2 cytokines. Data are representative of 3 independent experiments. (D) Flow cytometric analysis of surface receptors (CD25, CD28, and CD45RA) expression by CFSEloCD8+ T cells cultured for 6 days with allogeneic CD40L-activated dermal CD14+ DCs with a neutralizing IL-10 mAb or an isotype-matched control after reactivation with fresh autologous DCs for 24 hours before the analysis. Data are representative of 3 independent experiments. (E) Similar experiment as in panel D. Dot plots show the expression of granzyme B (top panel) and perforin (bottom panel) by the cultured viable CD3+CD8+ T cells. Data are representative of 3 independent experiments. (F) Granzyme B (top panel) and perforin (bottom panel) expression as measured by the cultured viable CD3+CD8+ T cells in 3 independent experiments; P = .01 and P = .06, respectively. (G) CD8+ T cells were cultured with autologous peptide-loaded in vitro HLA-A*0201+ CD14+ DCs in the presence of neutralizing anti–IL-10 mAb or an isotype-matched control for 10 days. CD40L and IL-7 were added on day 0, and IL-2 was added on day 3. Cells were restimulated for 24 hours with fresh DCs, and effector memory populations were analyzed by flow cytometry that was based on the costaining with CCR7 and CD45RA. Data are representative of 3 independent experiments.
Conversely, addition of neutralizing anti–IL-10 mAb to cocultures of dermal CD14+ DCs and allogeneic naive CD8+ T cells resulted in a detectable increased proportion of activated effector T cells (Figure 3D) expressing granzyme B and perforin (Figure 3E-F). Similar results were obtained with CD8+ T cells cocultured with in vitro–generated DC subsets (not shown). Furthermore, neutralizing IL-10 in cocultures of in vitro CD14+ DCs and naive CD8+ T cells enhanced the expansion of the (CCR7−CD45R−) effector memory CD8+ T cells (15.9% vs 5.8% and 4.1%; Figure 3G). Thus, IL-10 produced by CD14+ DCs limits their ability to prime effector CTLs.
IL-15 is localized to the DC–T-cell immunologic synapse
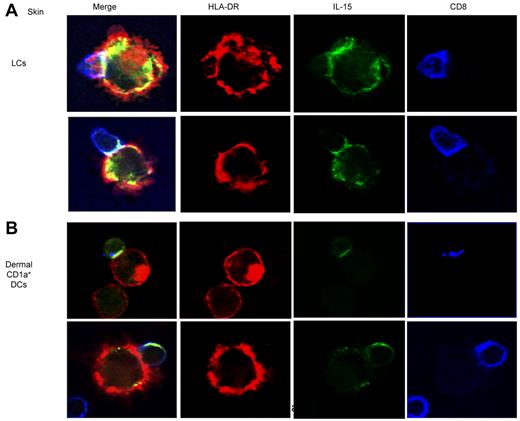
The partial inhibition of CD8+ T-cell priming induced by the anti–IL-15-Ab (Figure 2A) and the expression of IL-15/IL-15R complexes on the surface of APCs23 led us to consider whether IL-15 might be sequestered at the T-cell–APC interface. Indeed, confocal microscopic analysis performed at 12 hours of coculture of skin LCs and allogeneic naive CD8+ T cells found accumulation of IL-15 at the immunologic synapse (Figure 4 top panel). A close examination of conjugates also showed the presence of IL-15 within the CD8+ T cells which might reflect trogocytosis,24 a process whereby T cells internalize APC-derived vesicles. Similar findings have been observed when analyzing the activation of naive CD8+ T cells by dermal CD1a+ DCs (Figure 4 bottom panel). Taken together, our results indicate that DC-derived IL-15 concentrates at the immunologic synapse during CTL priming.
IL-15 concentrates in the DC–T-cell immunologic synapse. Skin LCs (top panel) or dermal CD1a+ DCs (bottom panel) were cocultured with purified allogeneic naive CD8+ T cells for 12 hours. CD40L was used to activate the DCs. Confocal immunofluorescence images show IL-15 accumulation (green) at the immunologic synapse that is generated between skin LCs (top panel) or dermal CD1a+ DCs (bottom panel) and naive CD8+ T cells. HLA-DR and CD8 are represented in red and blue, respectively. Data are representative of 3 independent experiments.
IL-15 concentrates in the DC–T-cell immunologic synapse. Skin LCs (top panel) or dermal CD1a+ DCs (bottom panel) were cocultured with purified allogeneic naive CD8+ T cells for 12 hours. CD40L was used to activate the DCs. Confocal immunofluorescence images show IL-15 accumulation (green) at the immunologic synapse that is generated between skin LCs (top panel) or dermal CD1a+ DCs (bottom panel) and naive CD8+ T cells. HLA-DR and CD8 are represented in red and blue, respectively. Data are representative of 3 independent experiments.
Discussion
We recently reported that human epidermal LCs are more potent than dermal CD14+ DCs at priming naive CD8+ T cells into potent CTLs. The present study was designed to identify the cellular mechanisms that confer LCs and CD14+ DCs with their differential capacity to initiate CD8+ T-cell immunity. The data highlight the role of IL-15 and IL-10 produced by LCs and dermal DCs, respectively.
Four complementary experimental approaches brought us to conclude that the production of IL-15 by LCs, but not CD14+ DCs, is critical to promote the differentiation of naive CD8+ T cells into granzyme B+ perforin+ CTLs. First, LCs, but not CD14+ DCs were found able to transcribe and secrete IL-15. In addition, CD1a+ dermal DCs, which are partial activators of CD8+ naive T cells,18 also transcribe IL-15. Second, the addition of neutralizing Abs to IL-15 (and its receptor) partially inhibit LC-mediated differentiation of naive CD8+ T cells into effector CTLs. Third, addition of IL-15 to cocultures of naive CD8+ T cells and dermal CD14+ DCs enhanced the cell ability to prime effector CD8+ T cells in a dose-dependent manner. Fourth, immunofluorescence studies that used confocal microscopy showed polarization of IL-15 and its receptor at the synapse between naive CD8+ T cells and both LCs and dermal CD1a+ DCs.
The CD8+ T cells primed by dermal CD14+ DCs and IL-15 showed an increased effector phenotype as measured by the level of granzymes, CD25, CCR7, and CD28, which were comparable with the levels observed by LCprimed CD8+ T cells. The antiapoptotic molecule Bcl-2 expression was also elevated in CD8+ T cells on priming with dermal CD14+ DCs and IL-15. This finding is consistent with observations in the mouse that IL-15 increases Bcl-2 expression on Ag-induced CD8+ T cells.25 The ultimate goal would be to test in vitro and in vivo in a clinical study whether CTLs primed in the presence of IL-15 will kill primary tumor cells as efficiently as they did cell lines.
Limited amounts of IL-15 presented by DCs were sufficient to drive the generation of effector CD8+ T cells. Indeed, preloading IL-15 onto the dermal CD14+ DCs before culturing with naive CD8+ T cells resulted in enhanced CD8+ T-cell proliferation and IFN-γ production compared with unloaded dermal CD14+ DCs. The effect was not due to IL-15 alone because the presence of DCs in the culture was required to mediate the observed IL-15 effect. Interestingly, IL-15 was dominant over IL-10 because the addition of soluble IL-15 to cocultures of dermal CD14+ DCs and naive CD8+ T cells overcame the inhibitory effect of IL-10 produced by the dermal CD14+ DC. In a similar manner, and consistent with the high expression of IL-15 by LCs, addition of IL-10 had only limited effect on CD8+ T-cell proliferation by LCs (supplemental Figures 2A and 3) However, the combination of IL-10 and TGF-β1 could antagonize the IL-15. Thus, our data are consistent with the earlier finding that IL-15 counteracts the inhibitory signaling pathway of TGF-β1.26
Although IL-15 Abs could potently inhibit the effect of exogenously added IL-15, they were only able to partially inhibit the IL-15 produced by LCs. Our confocal imaging studies indicated that IL-15 is being secreted directly into the immunologic synapse where it is probably protected from the neutralizing Ab. IL-15/IL-15Rα complexes are also known to be internalized and then recycled back to the surface.27 This mechanism of IL-15 stimulation might also be relatively resistant to IL-15 Ab treatment. Finally, it is also possible that the supplementation of our cultures with CD40L, IL-2, and IL-7 and the presence of costimulatory molecules CD80, CD86, or 4-1BBL on LCs also limit the efficacy of IL-15 inhibition.16
The CD14+ dermal DCs were found to transcribe TGF-β116 and to secrete large amounts of IL-10. IL-10 has broad anti-inflammatory properties, acting in a dual fashion on both T cells28,29 and APCs.21 In particular, IL-10 prevents CD8+ T cells from up-regulating the effector molecules granzyme and perforin. TGF-β1, a cytokine with immunoregulatory properties, also contribute to the specific effects of CD14+ DCs on CD8+ T-cell differentiation.30 Indeed, TGF-β1 delivers an opposing extrinsic signal to IL-15 to control the number of short-lived effector cells during clonal expansion and contraction in response to a viral infection.25,31 CD14+ DCs transcribe TGF-β1, although the protein could not be measured in DC supernatants by ELISA assay. In contrast to CD14+ DCs, LCs did not express any TGF-β1 transcripts. TGF-β1 acts in synergy with IL-10 to inhibit both the proliferation and effector molecule production by CD8+ T cells primed by LCs. TGF-β1 might be secreted by the dermal CD14+ DCs at low (undetectable by ELISA) levels,16 which are sufficient to enhance the regulatory effects of IL-10.
In conclusion, LCs and dermal CD1a+ DCs present IL-15 to naive CD8+ T cells at the immunologic synapse, resulting in potent CTL priming. In contrast dermal CD14+ DCs do not express IL-15 but express IL-10 and TGF-β1, which prevents the generation of effector CD8+ T cells. The current findings have several potentially important clinical implications. Studies have provided compelling evidence that inhibition of IL-10 and TGF-β1 signaling may address several key mechanisms of resistance and improve the efficacy of tumor immune therapy.29,32,33 In addition, the efficacy of IL-15 for eradicating tumors through enhanced induction of functional CD8+ cytotoxic T cells is now well documented in mouse tumor models.34,35 Our findings with human cells strongly support these studies and further provide a cellular mechanism by which blocking IL-10 and TGF-β signaling results in enhanced effector responses. It will be interesting to establish whether DCs that infiltrate tumors possess the properties of dermal CD14+ DCs. In this context, we and others have shown that the infiltration of tumors with DCs is able to induce T-cell differentiation in the Th2 pathway.36,37 Tumors might also be infiltrated by DC subtypes with the properties of dermal CD14+ DCs that would skew the differentiation of CD8+ T cells into noncytolytic T cells.38 Finally, the present study provides strong support for the delivery of Ag to IL-15–producing LCs, for induction of therapeutic Ag-specific CTL responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Elizabeth Trahan, Sebastien Coquery, Jennifer Shay, Jill Plants, Olivier Agouna-Deciat, and Amy O'Bar at the BIIR and Adiel Munk at Washington University School of Medicine Department of Pathology and Immunology for their help. They thank Dr Carson Harrod at the BIIR; Jennifer Duncan, surgeons, and nurses at the Baylor Medical Center Plastic Surgery Department; Dr Thomas Tung at the Washington University School of Medicine Department of Surgery; surgeons, nurses, and staff at the Barnes Jewish Hospital and Washington University School of Medicine for providing access to skin samples. They thank Dr Michael Ramsay and Dr Andrey Shaw for continuous support and critical reading of the manuscript.
This work was supported by the BHCS Foundation, the National Institutes of Health (grant RO-1 CA78846, RO-1 CA85540, PO-1 CA84512, U-19 AI-57234 to J.B.), the Washington University School of Medicine, Department of Pathology and Immunology (E.K.). J.B. held the W.W. Caruth Jr Chair for Transplantation Immunology Research.
National Institutes of Health
Authorship
Contribution: J.B. designed the research and wrote the manuscript; L.T.-S. contributed essential technical assistance and performed experiments; S.Z. generated critical new reagents and performed experiments; J.-P.B., S.C., and Y.C. contributed essential technical assistance; J.-P.G. designed the research relating to the cell biology content of the manuscript and made a figure; G.Z. designed the research and generated critical new reagents; and E.K. designed and performed the research, analyzed results, prepared the figures, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eynav Klechevsky, Department of Pathology and Immunology, Washington University School of Medicine, 425 S Euclid, St Louis, MO, 63110; e-mail: eklechevsky@path.wustl.edu; or Jacques Banchereau, Hoffmann-La Roche, 340 Kingsland St, Nutley, NJ, 07110; e-mail: jacques.banchereau@roche.com.