Abstract
The BH3-mimetic ABT-737 and an orally bioavailable compound of the same class, navitoclax (ABT-263), have shown promising antitumor efficacy in preclinical and early clinical studies. Although both drugs avidly bind Bcl-2, Bcl-xL, and Bcl-w in vitro, we find that Bcl-2 is the critical target in vivo, suggesting that patients with tumors overexpressing Bcl-2 will probably benefit. In human non-Hodgkin lymphomas, high expression of Bcl-2 but not Bcl-xL predicted sensitivity to ABT-263. Moreover, we show that increasing Bcl-2 sensitized normal and transformed lymphoid cells to ABT-737 by elevating proapoptotic Bim. In striking contrast, increasing Bcl-xL or Bcl-w conferred robust resistance to ABT-737, despite also increasing Bim. Cell-based protein redistribution assays unexpectedly revealed that ABT-737 disrupts Bcl-2/Bim complexes more readily than Bcl-xL/Bim or Bcl-w/Bim complexes. These results have profound implications for how BH3-mimetics induce apoptosis and how the use of these compounds can be optimized for treating lymphoid malignancies.
Introduction
Defects in the mitochondrial apoptotic pathway regulated by the Bcl-2 family of proteins play a major role in cancer development and in conferring chemoresistance.1 Within the family, Bax and Bak are essential for mitochondrial membrane permeabilization and cell death.2 Prosurvival proteins (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, A1) oppose Bax and Bak and ensure mitochondrial integrity and cell survival.1 These prosurvival proteins also interact with distant relatives that share only 1 Bcl-2 Homology region, BH3, that is critical for their proapoptotic function. The BH3-only proteins such as Bim, Bad, Puma, and Noxa act as stress sensors and relieve the inhibition of Bax and Bak by the prosurvival proteins.
The clinical efficacy of most anticancer therapeutics primarily reflects their ability to induce apoptosis. Resistance to conventional anticancer therapeutics (eg, etoposide) is often because of a failure to activate BH3-only proteins, for example because of mutation of the tumor suppressor p53, which is critical for transcriptional induction of Puma and Noxa after DNA damage.3 Overexpression of prosurvival Bcl-2 proteins, or silencing of BH3-only protein expression, are also associated with inferior therapeutic outcomes.4,5
BH3-mimetic drugs, such as ABT-737, were developed to directly counter such apoptotic blocks. ABT-737 binds avidly to Bcl-2, Bcl-xL, and Bcl-w, but not Mcl-1 or A1.6,7 In preclinical studies, it demonstrated single agent efficacy against tumors with low Mcl-1 or A1 levels, such as follicular lymphoma (FL), chronic lymphocytic leukemia (CLL), and small cell lung carcinoma (SCLC). ABT-737 shows limited toxicity toward normal cells, although there is transient reduction of platelets and lymphocytes.6,8 ABT-263 (navitoclax), an orally bioavailable compound in the same class with similar target specificity, also exhibited efficacy in various cancer-derived cell lines both in vitro and in vivo,6,9,10 and is undergoing phase I/II clinical trials with encouraging results.11-13
Overexpression of Bcl-2 or Bcl-xL has been implicated in the pathogenesis of lymphoid malignancies, and is frequently observed in CLL, acute lymphoblastic leukemia (ALL), FL, and diffuse large B-cell lymphoma (DLBCL).14-16 In some settings, elevated Bcl-2 or Bcl-xL expression directly correlates with poor responses to conventional therapies.5,17
Hence, direct targeting of Bcl-2 and Bcl-xL constitutes a promising approach for treating such malignancies. In this study, we exploited a substantial panel of gene-targeted mouse strains to address the mechanism of cell killing by ABT-737 and ABT-263 in normal and transformed lymphoid cells. Given both ABT-737 and ABT-263 target Bcl-xL, Bcl-2, and Bcl-w, we investigated whether these drugs kill by inhibiting each of these, or by inhibiting 1 or 2 preferentially. Our study reveals that Bcl-2 is the key target in lymphoid cells. Indeed, whereas overexpression of Bcl-2 sensitizes cells to killing by ABT-737, elevated expression of Bcl-xL or Bcl-w confers resistance instead.
Methods
Mice
Generation of vavP-Bcl-2,18 vavP-Mcl-1,19 Mcl-1fl/fl,8 Eμ-Myc,20 BimB/B, BimN/N and BimP/P,21 Bim−/−22 Puma−/−, Noxa−/−,23 Bmf−/−,24 Bax−/− (The Jackson Laboratory), Bak−/−, and Bax−/−Bak−/− mice2 were previously described. All animal experiments were performed in accordance with the guidelines of the Walter and Eliza Hall Institute (WEHI) Animal Ethics Committee.
Microarray and predictive model
IC50 values are the average of 3 independent experiments; curves were generated with a 4 parameter fit using XLFit. The mRNA expression levels of 10 Bcl-2 family members have been assessed by microarray, and correlated to resistance of 39 non-Hodgkin lymphoma (NHL) cells to ABT-263. The coefficient of correlation of each gene is represented in the linear predictor, ABT-263 Dx, with the positive sign indicating a resistant marker and the negative sign indicating a sensitive marker. The size of the coefficient of each gene correlates to its importance in the predictor. The linear predictor was generated with penalized lasso regression (cv.glmnet function in glmnet R package) based on 5-fold cross validation using the standardized expression intensities of the Bcl2 family members (Z scores). The ABT-263 Dx can be specified as: ABT-263 Dx = -0.07 + 0.27 ZMcl-1 − 0.6 ZBcl-2 − 0.14 ZNoxa − 0.05ZBmf.
The publicly available DLBCL patient dataset was downloaded from GEO database (GSE10846, HGU133plus2). The linear regression predictor was then applied to the Z-score standardized patient microarray data to predict the ABT-263-Dx score of each patient. The survival analysis was conducted using Kaplan-Meier curves, which plots the 3 evenly divided patient groups based on their ABT-263-Dx score, and Cox proportional hazard, which fits the Dx score as a continuous variable (survival, R package).
Both microarray datasets were normalized using Robust Multi-array Average.
Cell lines, FACS analysis, and cell survival assays
Murine DO11.10 T-hybridoma cells expressing murine Bcl-2 or Bcl-xL subcloned into the mammalian expression vector pEF-FLAG PGK puro25 were cultured in DMEM containing 10% FCS. Mcl-1fl/fl/Eμ-Myc lymphoma cells were isolated from 2 independent tumors and cultured in Iscove modified Dulbecco medium (IMDM) containing 20% FCS, 2mM l-glutamine, 100 ng/mL murine stem cell factor, 10 ng/mL IL-3 (Peprotech). These cells were transduced with pMSCV-IRES green fluorescent protein (GFP)–based expression vectors as described,7 treated with 4-OHT for 3 days then with dimethylsulfoxide (DMSO; 0.1%) or ABT-737 (Abbott) for a further 24 hours. For primary lymphocyte subpopulations, single-cell suspensions were prepared from thymus, spleens, or bone marrow (BM), and cultured with graded concentrations of ABT-737, ABT-263, etoposide, or dexamethasone. Cell viability was quantified by flow cytometric analysis of cells that excluded propidium iodide (PI) staining, 5 μg/mL. Results were normalized to the viability of cells that had been left untreated for 24 hours. Leukocyte subpopulations were identified by immunofluorescent staining with monoclonal antibodies coupled to fluorescein isothiocyanate, R-phycoerythrin (R-PE), allophycocyanin, followed by flow cytometric analysis: RA3-6B2 (anti-B220), 11-26C (anti-IgD), 333.12, or 5.1 (anti-IgM), YTS169 (anti-CD8), H129, or YTA321 (anti-CD4).
Hematopoietic reconstitution
Fetal liver cells from wild-type (WT) or Bim−/− embryos were harvested, cultured for 24 hours (in medium containing 100 ng/mL of stem cell factor, 10 ng/mL of IL-6, 10 ng/mL of Flt-3L, and 50 ng/mL of thrombopoietin) and infected with retroviruses encoding Bcl-xL, Bcl-2, Mcl-1 (human), or Bcl-w (mouse) in a pMSCV-IRES-GFP vector. Retrovirally transduced cells were injected into lethally irradiated (2 × 550 rads) recipient mice, and the hematopoietic system of reconstituted mice was analyzed 8 weeks later.
Immunoprecipitations and Western blotting
Coimmunoprecipitations were performed as previously described21 and used a rat mAb for Bim (3C5, Enzo Biosystems) or a hamster monoclonal anti–human Bcl-2 (3F11). Anti-FLAG (clone 9H1), anti-Bim (14A8 or 3C5; Alexis), anti-Bcl-2 (BD Pharmingen), anti–Bcl-xL (BD Bioscience), anti–Mcl-1 (Rockland), anti–Bcl-w (Stressgen), or anti-actin (Sigma-Aldrich) antibodies were used for Western blotting.
Affinity measurements
The affinity of recombinant mouse Bim, mouse NoxaA, BimNoxa, or human Noxa for mouse Bcl-2 or mouse Mcl-1 was determined by solution competition assay using a Biacore 3000 instrument, as previously described.26 Peptides were purchased from Mimotopes Pty. Peptide sequences were: mBim DLRPEIRIAQELRRIGDEFNETYTRR; BimNoxa DAELPPEFAAQLRKIGDKVYCTWTRR; mNoxaA RAELPPEFAAQLRKIGDKVYCTWSAP; hNoxa PAELEVECATQLRRFGDKLNFRQKLL.
Cellular protein redistribution assay
Statistics
Values used in heat maps are presented in supplemental Tables 1 and 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). They represent the mean of the EC50 collected from at least 3 independent experiments done in triplicate (except for Bax−/−/Bak−/− transgenic mice for which only 1 experiment could be done in triplicate). Statistical tests were performed on the Log-EC50 values rather than he EC50 values to uphold the assumption of normality. Two sample t tests comparing each transgenic to WT were performed where Log-EC50 values were available. P values were adjusted for multiple testing within a cell type using the Holm method.
Results
High Bcl-2 expression correlates with sensitivity to ABT-263
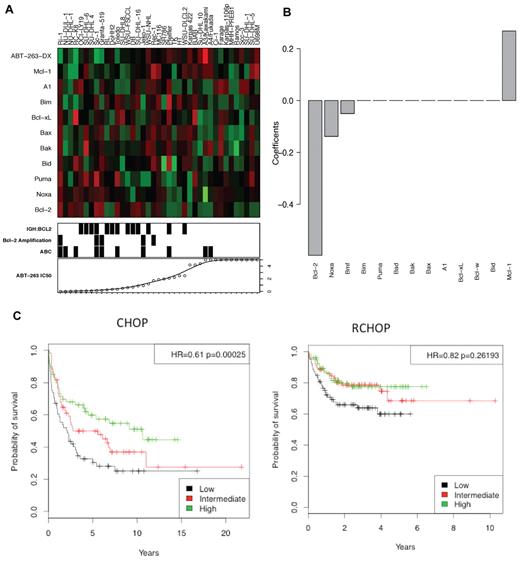
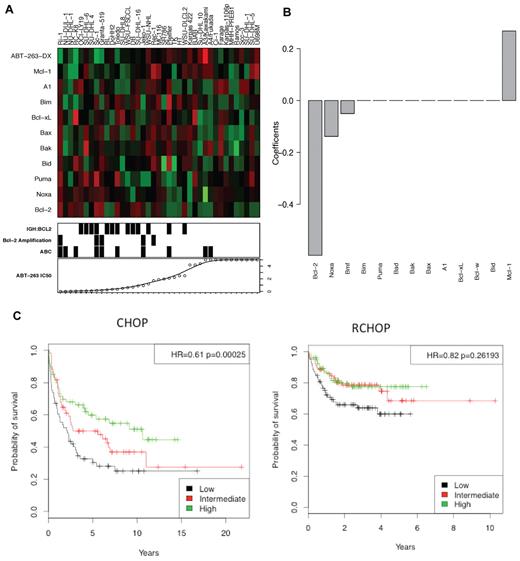
To clarify further which Bcl-2 family members determine sensitivity of lymphoma cells to ABT-263, we analyzed the sensitivity of 39 human NHL cell lines in relation to the expression of 10 Bcl-2 family members, as determined by microarray analysis (Figure 1A). High expression of BCL-2 and NOXA mRNAs proved the strongest predictor of ABT-263 sensitivity (Figure 1B), as previously reported.15,29-31 In contrast, high MCL-1 expression was a strong indicator of resistance to ABT-263, in agreement with studies with diverse cell lines.7,29,32 Expression of the other genes tested (BID, PUMA, BAX, BAK, BIM, BAD, BCL-X, BCL-W, or A1) did not significantly correlate with ABT-263 sensitivity.
High Bcl-2 expression correlates with sensitivity to ABT-263. The level of expression of 10 Bcl-2 family members in 39 NHL cell lines was assayed by microarray. (A) The heat-map represents the mRNA expression levels of Bcl-2 family genes. Red represents high expression, green low expression. ABT-263-DX is the linear predictor of cell line resistance against ABT-263 (see “Microarray and predictive model”). The bottom panel displays ABT-263 IC50 values, presence of the t(14:18) translocation (IGH:BCL2) or amplification of Bcl-2 (> 3 copies) and ABC subtype classification. (B) The coefficients of the Bcl-2 family genes have been represented as a linear predictor. The value of each coefficient represents its relative contribution to the predictive signature. (C) In patients treated with R-CHOP and CHOP,33 a low ABT-263-DX value correlated with poor prognosis. The corresponding hazard ratios and P values were calculated using Cox regressing model fitting the ABT-263-DX value as a continuous variable.
High Bcl-2 expression correlates with sensitivity to ABT-263. The level of expression of 10 Bcl-2 family members in 39 NHL cell lines was assayed by microarray. (A) The heat-map represents the mRNA expression levels of Bcl-2 family genes. Red represents high expression, green low expression. ABT-263-DX is the linear predictor of cell line resistance against ABT-263 (see “Microarray and predictive model”). The bottom panel displays ABT-263 IC50 values, presence of the t(14:18) translocation (IGH:BCL2) or amplification of Bcl-2 (> 3 copies) and ABC subtype classification. (B) The coefficients of the Bcl-2 family genes have been represented as a linear predictor. The value of each coefficient represents its relative contribution to the predictive signature. (C) In patients treated with R-CHOP and CHOP,33 a low ABT-263-DX value correlated with poor prognosis. The corresponding hazard ratios and P values were calculated using Cox regressing model fitting the ABT-263-DX value as a continuous variable.
Based on these observations, we developed a linear predictor, denoted ABT-263-DX (see “Microarray and predictive model” for formula), to model correlations between gene expression and sensitivity to ABT-263, which we validated using a publicly available dataset (GSE10846)33 for human DLBCL. Patients in this cohort had received a standard combination chemotherapy of cyclophosphamide, doxorubicin, vincristine, and prednisone, with (R-CHOP) or without rituximab (CHOP).33 In CHOP-treated DLBCL patients, low ABT-263-DX values (predicting high sensitivity to ABT-263) were strongly associated with inferior outcomes (Figure 1C, HR = 0.61, P = .00025). Notably, this association was largely lost (P = .04) when the data were adjusted for lymphoma subtype (germinal center B cell–like: GCB versus activated B cell–like: ABC),34 suggesting a probable correlation between ABT-263-DX and GCB/ABC subtype. A similar, albeit smaller, trend was also evident in R-CHOP–treated DLBCL patients (Figure 1C, HR = 0.82, P = .026).
Taken together, these analyses suggest that the DLBCL patients most probable to respond to ABT-263 are those who fare poorly with conventional standard-of-care regimens.
High Bcl-2 levels also sensitize normal cells to ABT-263 and ABT-737
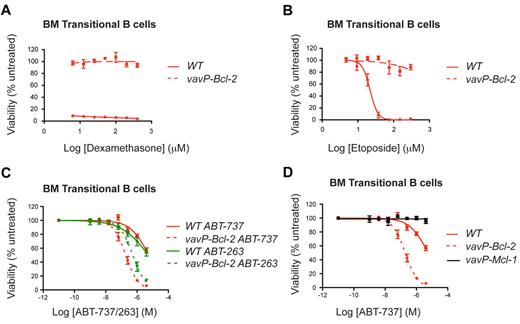
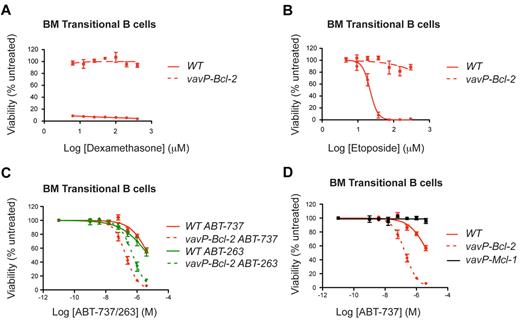
As elevated BCL-2 expression correlated with sensitivity to ABT-263 in lymphoma cells (Figure 1), we investigated whether increased Bcl-2 also sensitizes their nontransformed counterparts. We focused on vavP–Bcl-2 transgenic mice, which overexpress human BCL-2 in all nucleated hematopoietic cells.18 As expected,18 Bcl-2 overexpression rendered vavP–Bcl-2 lymphocytes highly resistant to lymphotoxic agents such as dexamethasone (Figure 2A) and etoposide (Figure 2B).
High Bcl-2 expression protects lymphoid cells against dexamethasone or etoposide, but sensitizes to ABT-737/263. BM transitional B cells from WT and vavP–Bcl-2 transgenic mice were isolated and cultured in the presence of (A) dexamethasone, (B) etoposide, (C) ABT-737, or ABT-263 at the indicated doses for 24 hours. (D) BM transitional B cells from vavP–Mcl-1 mice are resistant to ABT-737. Survival data are shown for transitional B cells (n = 3 mice for each genotype) and are the results of 3 independent experiments, each performed in triplicate. Experiments depicted in panels C and D were performed concurrently. Values represent mean ± SEM.
High Bcl-2 expression protects lymphoid cells against dexamethasone or etoposide, but sensitizes to ABT-737/263. BM transitional B cells from WT and vavP–Bcl-2 transgenic mice were isolated and cultured in the presence of (A) dexamethasone, (B) etoposide, (C) ABT-737, or ABT-263 at the indicated doses for 24 hours. (D) BM transitional B cells from vavP–Mcl-1 mice are resistant to ABT-737. Survival data are shown for transitional B cells (n = 3 mice for each genotype) and are the results of 3 independent experiments, each performed in triplicate. Experiments depicted in panels C and D were performed concurrently. Values represent mean ± SEM.
In striking contrast, multiple lymphoid subpopulations isolated from the thymi or BM of vavP–Bcl-2 transgenic mice were markedly more, not less, sensitive to ABT-263 or ABT-737 (Figure 2C). This was most evident for the lymphoid populations normally resistant to these BH3-mimetics, such as BM transitional B cells (IgMhiIgDmed) or immature B cells (IgMhiIgDlo). This sensitization is not a general consequence of prosurvival protein overexpression because similar cells isolated from vavP–Mcl-1 transgenic mice19 were highly resistant to ABT-737 (Figure 2D). Treatment with ABT-737 did not induce a detectable change in the expression of Bcl-2, Bim, Mcl-1, or Bcl-xL in the cells analyzed (supplemental Figure 1).
Loss of Bim, or combined absence of Bax and Bak, renders lymphoid cells resistant to ABT-737
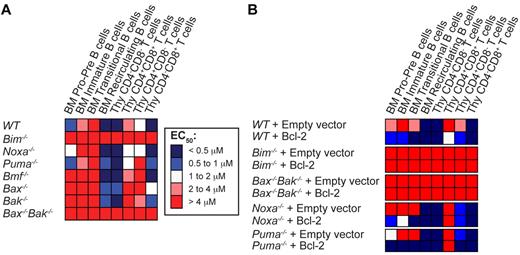
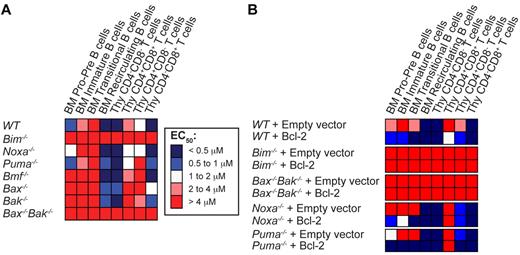
We next studied the impact on ABT-737–induced cell death when Bim, Noxa, Puma, or Bmf are deleted because the corresponding BH3-only proteins are key regulators of lymphoid apoptosis.22 Whereas loss of Noxa, Puma or Bmf had minimal impact on sensitivity to ABT-737, loss of Bim rendered all lymphoid sub-populations examined highly resistant to ABT-737 (Figure 3A; see supplemental Table A for actual values). To ascertain whether Bim is also critical for ABT-737–induced cell death in Bcl-2–overexpressing cells, we transduced fetal liver cells of different genotypes with control or Bcl-2–expressing retroviral vectors and reconstituted the hematopoietic system of lethally irradiated mice with the infected cells. Strikingly, loss of Bim, but not Noxa or Puma, completely abrogated the enhanced sensitivity to ABT-737 afforded by Bcl-2 overexpression (Figure 3B).
Bim plays an important role in the response to ABT-737. (A) Thymocytes and BM-derived B cells from wt, Bim−/−, Noxa−/−, Puma−/−, Bmf−/−, Bax−/−, Bak−/−, or Bax−/−Bak−/− mice were cultured in the absence or presence of increasing doses of ABT-737, and their viability determined after 24 hours. EC50 values are represented as a heat map. Blue represents sensitivity, and red, resistance. (B) The hematopoietic system of lethally irradiated mice was reconstituted with WT, Bim−/−, Noxa−/−, Puma−/−, or Bax−/−Bak−/− fetal liver cells retrovirally transduced with an empty vector or a vector encoding Bcl-2. Eight weeks after reconstitution, the viability of the resulting cells in response to treatment with several doses of ABT-737 was analyzed in culture as described in Figure 2; n = 3 mice per group, 3 independent experiments (except for Bax−/−Bak−/− n = 1 because of the difficulty to obtain viable animals). Values represent the mean of EC50 ± SEM, detailed in supplemental Table 2.
Bim plays an important role in the response to ABT-737. (A) Thymocytes and BM-derived B cells from wt, Bim−/−, Noxa−/−, Puma−/−, Bmf−/−, Bax−/−, Bak−/−, or Bax−/−Bak−/− mice were cultured in the absence or presence of increasing doses of ABT-737, and their viability determined after 24 hours. EC50 values are represented as a heat map. Blue represents sensitivity, and red, resistance. (B) The hematopoietic system of lethally irradiated mice was reconstituted with WT, Bim−/−, Noxa−/−, Puma−/−, or Bax−/−Bak−/− fetal liver cells retrovirally transduced with an empty vector or a vector encoding Bcl-2. Eight weeks after reconstitution, the viability of the resulting cells in response to treatment with several doses of ABT-737 was analyzed in culture as described in Figure 2; n = 3 mice per group, 3 independent experiments (except for Bax−/−Bak−/− n = 1 because of the difficulty to obtain viable animals). Values represent the mean of EC50 ± SEM, detailed in supplemental Table 2.
Overexpression of Bcl-2 causes Bim accumulation
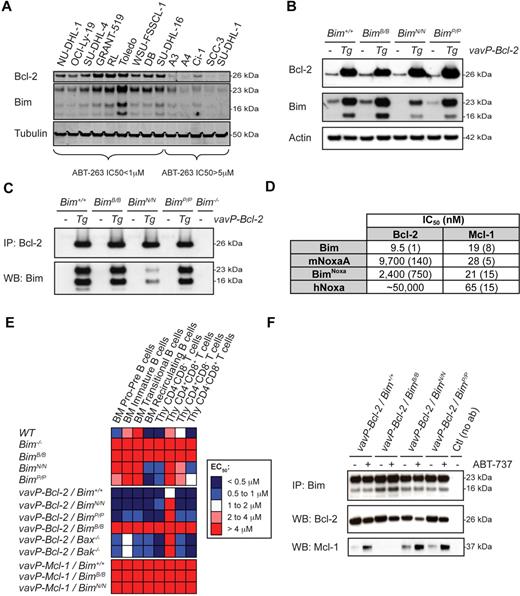
These studies demonstrate the critical role of Bim in ABT-737–induced apoptosis. Because Bim mRNA expression did not predict sensitivity to ABT-263 (Figure 1), we next examined the expression of Bim protein in 14 of the NHL cell lines classified as sensitive (IC50 < 1μM) or resistant (IC50 ≥ 5μM) to ABT-263. Notably, all ABT-263–sensitive cell lines exhibited higher levels of Bim and Bcl-2 than the resistant lines (Figure 4A), consistent with earlier observations.15 Bim protein levels were also markedly higher in vavP–Bcl-2 lymphocytes than their WT counterparts (Figure 4B). Moreover, the half-life of Bim was significantly extended in vavP–Bcl-2 compared with WT cells (supplemental Figure 2A). These observations suggest that binding to Bcl-2 protects Bim from degradation.
Sensitization of vav-Bcl-2 lymphocytes to ABT-737 requires the binding of Bim to Mcl-1. (A) Western blot analysis of NHL cell lines having higher or lower levels of Bcl-2 RNA by microarray analysis (Figure 1). Those expressing higher levels of Bcl-2 protein are sensitive to ABT-263 and express higher levels of Bim. (B) Expression of Bcl-2 and Bim in vavP–Bcl-2/Bim+/+, vavP–Bcl-2/BimB/B, vavP–Bcl-2/BimN/N, and vavP–Bcl-2/BimP/P thymocytes was determined by Western blotting. (C) Thymocyte lysates were prepared and the protein complexes were analyzed by immunoprecipitation and Western blotting. (D) Affinities of Bim and Noxa BH3 peptides for mouse Bcl-2 and mouse Mcl-1 were determined by solution competition assay using a Biacore optical biosensor. Numbers in brackets represent standard deviations for n = 2 to 4 experiments. (E) The sensitivity of BM-derived B cells and thymocytes of the indicated genotypes to ABT-737 was measured and data are represented in a heat map as described in Figure 2. Results represent the mean of at least 3 independent experiments per genotype (detailed in supplemental Table 1). Restricting the binding specificity of Bim to that of Bad renders all cell types resistant to ABT-737, regardless of Bcl-2 overexpression. As a control, the BH3 mutations in Bim did not affect the resistance of vavP-Mcl-1–transgenic cells to ABT-737–induced cell death (vavP–Mcl-1/BimB/B and vavP–Mcl-1/BimN/N). (F) Thymocytes from vavP–Bcl-2/Bim+/+ mice were treated with ABT-737 (1μM) for 5 hours before Bim immunoprecipitation. The composition of the protein complexes was analyzed by Western blotting.
Sensitization of vav-Bcl-2 lymphocytes to ABT-737 requires the binding of Bim to Mcl-1. (A) Western blot analysis of NHL cell lines having higher or lower levels of Bcl-2 RNA by microarray analysis (Figure 1). Those expressing higher levels of Bcl-2 protein are sensitive to ABT-263 and express higher levels of Bim. (B) Expression of Bcl-2 and Bim in vavP–Bcl-2/Bim+/+, vavP–Bcl-2/BimB/B, vavP–Bcl-2/BimN/N, and vavP–Bcl-2/BimP/P thymocytes was determined by Western blotting. (C) Thymocyte lysates were prepared and the protein complexes were analyzed by immunoprecipitation and Western blotting. (D) Affinities of Bim and Noxa BH3 peptides for mouse Bcl-2 and mouse Mcl-1 were determined by solution competition assay using a Biacore optical biosensor. Numbers in brackets represent standard deviations for n = 2 to 4 experiments. (E) The sensitivity of BM-derived B cells and thymocytes of the indicated genotypes to ABT-737 was measured and data are represented in a heat map as described in Figure 2. Results represent the mean of at least 3 independent experiments per genotype (detailed in supplemental Table 1). Restricting the binding specificity of Bim to that of Bad renders all cell types resistant to ABT-737, regardless of Bcl-2 overexpression. As a control, the BH3 mutations in Bim did not affect the resistance of vavP-Mcl-1–transgenic cells to ABT-737–induced cell death (vavP–Mcl-1/BimB/B and vavP–Mcl-1/BimN/N). (F) Thymocytes from vavP–Bcl-2/Bim+/+ mice were treated with ABT-737 (1μM) for 5 hours before Bim immunoprecipitation. The composition of the protein complexes was analyzed by Western blotting.
To test this further, we crossed vavP–Bcl-2 mice with Bim-BH3 “knock-in” mice (BimB/B, BimN/N, or BimP/P) that express mutant Bim proteins that have had their native BH3 domain replaced with that of mouse Bad, mouse NoxaA, or mouse Puma (BimBad, BimNoxa, and BimPuma) and exhibit their binding specificities.21 Consistent with the notion that binding to Bcl-2 is necessary to drive Bim accumulation in lymphoid cells expressing the vavP–Bcl-2 transgene, BimBad and BimPuma were as elevated as WT Bim (Figure 4B). BimNoxa levels were elevated to a lesser degree, reflecting the lower affinity of native NoxaA for Bcl-2.35 The strong association of Bcl-2 with WT Bim, BimBad and BimPuma was confirmed by coimmunoprecipitation (Figure 4C). It is notable that the weak affinity of BimNoxa for Bcl-2 (Figure 4D), which was barely detectable in BimN/N lymphocytes (Figure 4B), is sufficient to allow accumulation of Bcl-2/BimNoxa complexes when Bcl-2 is highly expressed. By contrast, WT Bim and BimNoxa were elevated in the vavP–Mcl-1 transgenic cells (supplemental Figure 2B), whereas the level of BimBad, which does not bind Mcl-1,21 was comparable with cells from control mice. Taken together, these results indicate that complex formation with prosurvival proteins significantly prolongs the half-life of Bim.
ABT-737–induced cell death requires the inhibition of endogenous Mcl-1 by Bim
To clarify how Bim triggers apoptosis in Bcl-2–overexpressing cells treated with ABT-737, we studied the sensitivity of lymphoid cells from vavP–Bcl-2-/BimB/B, vavP–Bcl-2/BimN/N, and vavP–Bcl-2/BimP/P mice. Strikingly, BimB/B cells were very resistant to ABT-737, regardless of Bcl-2 expression (Figure 4E, supplemental Table 1). In contrast, vavP–Bcl-2/BimN/N and vavP–Bcl-2/BimP/P lymphocytes were as sensitive to ABT-737 as vavP–Bcl-2/Bim+/+ cells. The dramatic differences can be explained by the fact that both BimNoxa and BimPuma can bind Mcl-1, but BimBad cannot. Notably, the level of Bcl-2 bound to Bim or the Bim mutants consistently decreased after ABT-737 treatment, whereas the level of Mcl-1 complexed with Bim or its mutants increased, except as anticipated in BimBad-expressing cells (Figure 4F).
To confirm that the changes in protein-protein association after ABT-737 treatment result from displacement of proteins from preexisting complexes rather than engagement of newly synthesized proteins, we performed Bim immunoprecipitation after 5-hour treatment in the presence of cycloheximide. Even without protein synthesis, ABT-737 treatment reduced association of Bim with Bcl-2 and enhanced binding to Mcl-1 (supplemental Figure 2C). However, this does not exclude the possibility that newly synthesized Bim and Mcl-1 interact preferentially when Bcl-2 is occupied by ABT-737.
Collectively, these findings demonstrate that for ABT-737 to trigger killing of Bcl-2–overexpressing lymphoid cells, Bim must be available to neutralize Mcl-1, which is not directly targeted by ABT-737.
Overexpression of Bcl-xL or Bcl-w, unlike Bcl-2, does not confer sensitivity to ABT-737
Because ABT-737 binds purified recombinant Bcl-2 and Bcl-xL proteins with similar affinities,6,7 it was surprising that the level of BCL-XL expression did not correlate with the sensitivity of NHL cells to ABT-263 (Figure 1). To investigate this further, we reconstituted the hematopoietic system of lethally irradiated mice with WT fetal liver cells infected with retroviruses encoding FLAG-tagged Bcl-2, Bcl-xL, Bcl-w, or Mcl-1. We confirmed that all 4 proteins were functionally overexpressed, clearly protecting against etoposide and dexamethasone (supplemental Figure 3A).
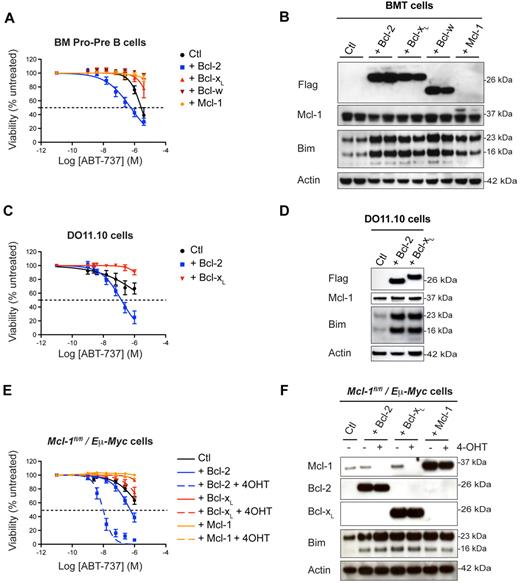
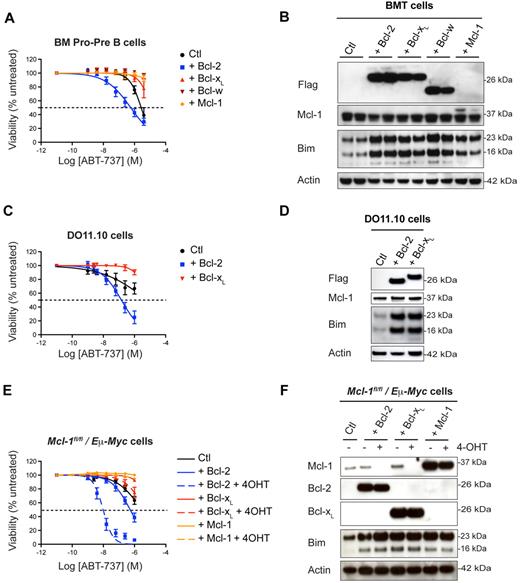
In the experiments described above (Figure 2), overexpression of Bcl-2 sensitized cells to ABT-737 treatment, whereas overexpression of Mcl-1 conferred resistance (Figure 5A-B). In surprising contrast to the effect of Bcl-2 excess, overexpressed Bcl-xL and Bcl-w induced dramatic ABT-737 resistance in all BM and thymic lymphoid subsets (Figure 5A, supplemental Figure 3B, and supplemental Table 2 for values). As with Bcl-2 and Mcl-1, overexpression of Bcl-xL or Bcl-w elevated levels of Bim (Figure 5B), which was sequestered in complexes with these prosurvival proteins (supplemental Figure 4A). High Bcl-2, Bcl-xL, or Bcl-w levels protected Bim from degradation (supplemental Figure 4B) but did not increase Bim mRNA levels (supplemental Figure 4C). These results were confirmed in the T-cell hybridoma line DO11.10. Overexpression of Bcl-2 sensitized DO11.10 cells to ABT-737, whereas overexpression of Bcl-xL protected them against this compound (Figure 5C), even though each augmented the levels of Bim comparably (Figure 5D).
Bcl-xL and Bcl-w overexpression protects against ABT-737 induced cell death. (A) WT fetal liver cells retrovirally transduced with pMIG–Bcl-2, pMIG–Bcl-xL, pMIG–Bcl-w, pMIG-Mcl1, or control pMIG vectors were transplanted into lethally irradiated recipient mice (n = 5 per group). Lymphocytes were harvested 8 weeks later and treated in culture with ABT-737. The reminder of the results are shown as a heat map in supplemental Figure 3A. (B) Overexpression of Bcl-2, Bcl-xL, Bcl-w, or Mcl-1 in lymphocytes is accompanied by an increase in Bim as assessed by Western blotting. For a reason that is unclear, the Flag-tagged version of Mcl-1 is very difficult to detect with our anti-Flag antibody, but is detected with the anti–Mcl-1 antibody as a slower moving band. (C) Murine DO11.10 T hybridoma cells stably transfected with pEF–mBcl-2, pEF–mBcl-xL or control pEF vectors were challenged with increasing concentrations of ABT-737 for 24 hours. (D) Increase in Bim levels on overexpression of Bcl-2 or Bcl-xL in DO11.10 cells as revealed by Western blotting. (E) Mcl-1fl/fl/Eμ-Myc lymphoma cells retrovirally transduced with pMIG–Bcl-2, pMIG–Bcl-xL, pMIG–Mcl-1 or control pMIG vectors were treated for 3 days with 4-OHT to remove endogenous Mcl-1 before being exposed to ABT-737. These experiments have been reproduced using an independent Mcl-1fl/fl/Eμ-Myc lymphoma line. (F) The corresponding levels of Mcl-1, Bcl-2, Bcl-xL, and Bim were determined by Western blotting.
Bcl-xL and Bcl-w overexpression protects against ABT-737 induced cell death. (A) WT fetal liver cells retrovirally transduced with pMIG–Bcl-2, pMIG–Bcl-xL, pMIG–Bcl-w, pMIG-Mcl1, or control pMIG vectors were transplanted into lethally irradiated recipient mice (n = 5 per group). Lymphocytes were harvested 8 weeks later and treated in culture with ABT-737. The reminder of the results are shown as a heat map in supplemental Figure 3A. (B) Overexpression of Bcl-2, Bcl-xL, Bcl-w, or Mcl-1 in lymphocytes is accompanied by an increase in Bim as assessed by Western blotting. For a reason that is unclear, the Flag-tagged version of Mcl-1 is very difficult to detect with our anti-Flag antibody, but is detected with the anti–Mcl-1 antibody as a slower moving band. (C) Murine DO11.10 T hybridoma cells stably transfected with pEF–mBcl-2, pEF–mBcl-xL or control pEF vectors were challenged with increasing concentrations of ABT-737 for 24 hours. (D) Increase in Bim levels on overexpression of Bcl-2 or Bcl-xL in DO11.10 cells as revealed by Western blotting. (E) Mcl-1fl/fl/Eμ-Myc lymphoma cells retrovirally transduced with pMIG–Bcl-2, pMIG–Bcl-xL, pMIG–Mcl-1 or control pMIG vectors were treated for 3 days with 4-OHT to remove endogenous Mcl-1 before being exposed to ABT-737. These experiments have been reproduced using an independent Mcl-1fl/fl/Eμ-Myc lymphoma line. (F) The corresponding levels of Mcl-1, Bcl-2, Bcl-xL, and Bim were determined by Western blotting.
To determine whether the opposing effects of Bcl-2 and Bcl-xL on ABT-737–induced apoptosis hold in a tumor model, we used the Eμ-Myc transgenic mice. Eμ-Myc–driven lymphomas exhibit a high degree of resistance to ABT-737, because of high levels of Mcl-1.36 We generated lymphomas in Eμ-Myc/Mcl-1flox/flox/RosaCreER mice, so that Mcl-1 could be deleted at will by 4-hydroxytamoxifen (4-OHT) treatment. Overexpression of Bcl-2 in Eμ-Myc/Mcl-1flox/flox/RosaCreER cells sensitized these lymphoma cells to in vitro treatment with ABT-737, especially when endogenous Mcl-1 was removed (Figure 5E-F). In contrast, Bcl-xL or Mcl-1–overexpressing cells were resistant to ABT-737, even in the absence of endogenous Mcl-1 (Figure 5E-F). These data are consistent with our findings in human NHL cells. Overall, our conclusion is that ABT-737 is much more efficient at killing cells that overexpress Bcl-2 than it is at killing cells that overexpress Bcl-xL or Bcl-w (Figure 1B).
ABT-737 releases Bim from sequestration by Bcl-2 more readily than Bim bound to Bcl-xL or Bcl-w
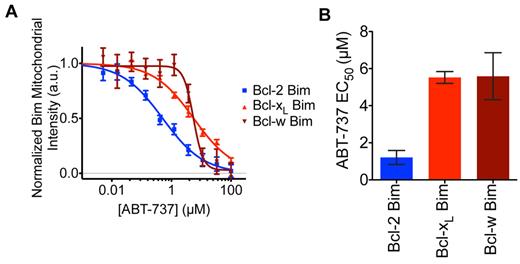
To fully investigate why overexpressing Bcl-2, but not Bcl-xL or Bcl-w, sensitizes cells to ABT-737 even though it binds all of them in vitro,6,7 we tested the susceptibility of their cellular complexes to ABT-737 treatment. In this cellular redistribution assay,28 mCherry-tagged BimSΔC (a mutant lacking the C-terminal membrane targeting domain) was sequestered to the mitochondria by binding to eGFP-tagged full-length Bcl-2, Bcl-xL, or Bcl-w (Figure 6). On addition of ABT-737, mCherry BimSΔC was displaced from the mitochondria in a time-dependent manner (Figure 6). Strikingly, ABT-737 was ∼ 5-fold more potent at displacing Bim from Bcl-2 than from Bcl-xL or Bcl-w. The 6-fold higher affinity of Bim for Bcl-xL than for Bcl-2 that we previously reported37 probably contributes to this difference. Thus, the differential impact of ABT-737 on cells with elevated Bcl-2, Bcl-xL, or Bcl-w can be accounted for by its differential ability to disrupt cellular complexes of Bim with these prosurvival proteins (supplemental Figure 6).
ABT-737 exhibits greater potency at displacing BimSΔC from Bcl-2 than from Bcl-xL or Bcl-w. Flp-In T-REx 293 cells with single copy integration of an inducible bicistronic construct expressing mCherry-BimSΔC and eGFP-Bcl-2, Bcl-xL or Bcl-w were imaged live every 25 minutes on treatment of ABT-737 at a 10-point dose range from 5nM to 100μM. (A) Displacement of mCherry-BimSΔC from the mitochondria was measured at 350 minutes after compound addition and normalized to DMSO controls at t = 0. (B) EC50 values were estimated by fitting mitochondrial displacement of mCherry-BimSΔC in response to ABT-737 to sigmoid curves and averaged for 2 to 4 biologic replicates. P values comparing Bcl-2 with Bcl-xL and Bcl-w were .0009 and .028, respectively. Error bars represent SE, and P values were calculated using a Student t test.
ABT-737 exhibits greater potency at displacing BimSΔC from Bcl-2 than from Bcl-xL or Bcl-w. Flp-In T-REx 293 cells with single copy integration of an inducible bicistronic construct expressing mCherry-BimSΔC and eGFP-Bcl-2, Bcl-xL or Bcl-w were imaged live every 25 minutes on treatment of ABT-737 at a 10-point dose range from 5nM to 100μM. (A) Displacement of mCherry-BimSΔC from the mitochondria was measured at 350 minutes after compound addition and normalized to DMSO controls at t = 0. (B) EC50 values were estimated by fitting mitochondrial displacement of mCherry-BimSΔC in response to ABT-737 to sigmoid curves and averaged for 2 to 4 biologic replicates. P values comparing Bcl-2 with Bcl-xL and Bcl-w were .0009 and .028, respectively. Error bars represent SE, and P values were calculated using a Student t test.
Discussion
The recently completed phase 1 trials12,13,38 have established the safety and optimal dosing of navitoclax (ABT-263). Significant objective responses were achieved in 30%-50% of patients with CLL12,13 but not in patients with other lymphoid neoplasms12 or SCLC,38 despite encouraging preclinical activity of ABT-263 in all these tumors.12,38 To better understand determinants of ABT263/ABT-737 activity, we conducted detailed mechanism-of-action studies in cell lines as well as primary mouse lymphocytes isolated from a large panel of mouse strains that harbor mutations in one or more Bcl-2 family member. Our focus here is on lymphoid compartment because lymphoid malignancies12,13 are the most promising indications for ABT-263 therapy.
Consistent with recent studies demonstrating that Bcl-2 levels correlate with sensitivity to ABT-737,7,15 we found that high BCL-2 was the best predictor of sensitivity to ABT-263 in a panel of 39 NHL cell lines. Our data thus provide support of why ABT263 may have such benefit in CLL as these tumors have minimal to no BCL-xL.39 In accord with this, our initial studies revealed that the lymphoid subpopulations known to express low levels of Bcl-2, such as immature B cells, are insensitive to ABT-737. Notably, the resistant subpopulations were markedly sensitized to ABT-737 by overexpression of Bcl-2, which in contrast provided dramatic protection against other chemotherapeutics.18
Why are Bcl-2–overexpressing cells more sensitive to ABT-737? At first glance, this is counterintuitive because in most cases, increasing the amount of the drug target should raise the barrier to cytotoxicity. Our studies confirm that ABT-737 triggered Bax/Bak-dependent apoptosis and establish definitively that increased Bim is the key driver of ABT-737 lymphotoxicity, in general agreement with previous studies in cell lines15,40 and the central role of Bim in lymphoid homeostasis.22 Overexpression of any prosurvival Bcl-2 family protein caused the accumulation of Bim in nontransformed as well as malignant lymphoid cells, confirming that the formation of complexes with prosurvival proteins is an important mechanism regulating the steady state level of Bim.41,42 We show that Bcl-2/Bim complexes are highly susceptible to disruption by ABT-737, and that the released Bim then binds to Mcl-1. It is also conceivable that some of the Bim released from Bcl-2 also directly activates Bax or Bak. Our studies with Bim mutants indeed revealed that the 3 forms of Bim able to bind Mcl-1 with high affinity (WT Bim, BimPuma or BimNoxa) were able to kill lymphoid cells on ABT-737 treatment, whereas the one form (BimBad) that binds Mcl-1 poorly was associated with complete resistance, regardless of whether Bcl-2 is overexpressed. The lack of a good antibody against A1 prevented us from considering it further. However, we do not exclude that A1 may play a similar role as Mcl-1 in certain subpopulations, because it was shown to be up-regulated, together with Bcl-xL, in response to cell-cell interactions within the lymph node microenvironment and be the reason of acquired resistance to ABT-737.43
Perhaps the most important and unexpected outcome from our study, confirmed in different lymphoid cell types, is that increasing Bcl-2, but not Bcl-xL or Bcl-w, sensitizes cells to ABT-737 even though it binds tightly to all 3 in cell-free assays.6,7,44 Of note, protection of lymphoid cells from ABT-737 by Bcl-w was previously reported,45 although this study also reported that both Bcl-2 and Bcl-xL–overexpressing Eμ-Myc lymphoma cells were sensitive to ABT-737. Interestingly, CD4+8+ thymocytes, which are highly sensitive to many apoptotic stimuli (eg, DNA damage, glucocorticoids)46 were the cell type most resistant to ABT-737 treatment in WT mice. We can now account for this observation, as CD4+8+ thymocytes depend on Bcl-xL for their survival47 and ABT-737 appears to be relatively inefficient at disrupting Bcl-xL/Bim complexes. Consistent with this notion, we recently observed that exposure of Mcl-1–deficient fibroblasts to increasing doses of ABT-737 selected for the emergence of resistant clones that had markedly up-regulated Bcl-xL, but not for cells with increased Bcl-2 (our unpublished observations).
Interestingly, the primary dose-limiting toxicity of ABT-263 (recapitulated by ABT-737 in mice) is thrombocytopenia because of Bcl-xL antagonism.8,13 It thus appears that although these BH3-mimetics target Bcl-xL with sufficient avidity to kill platelets, they are not efficient antagonists of Bcl-xL in lymphoid or leukemic cells. This probably reflects the importance of Bad and Bak, compared with Bim, for platelet survival48 ; indeed, ABT-737 is more efficient at disrupting Bad/Bcl-xL complexes than Bim/Bcl-xL complexes in cells (supplemental Figure 5). Sensitivity or resistance to ABT-737 thus depends on the composition of the key complex formed between the predominant prosurvival and proapoptotic proteins in a given cell.
The differential impact of compounds like ABT-737 on Bcl-2 and Bcl-xL is highly relevant for cancers because elevation of Bcl-xL is closely linked to chemoresistance49 including to ABT-737.43 Our studies presage the requirement to identify the critical complex that needs to be targeted by BH3-mimetics for optimal cell killing. Whereas elevated levels of Bim/Bcl-2 complexes indicate sensitivity to ABT-737 in lymphoid cells (this study) and certain breast tumors,50 high levels of Bim/Bcl-xL complexes are associated with resistance. Our results thus highlight the importance of determining the ability of BH3-mimetics at disrupting complexes between pro and antiapoptotic proteins in vivo, which might not reflect their affinity for prosurvival proteins in vitro. The strategy to achieve high kill rates will then depend on choosing the optimal BH3-mimetic to target the key complex in each tumor. Although these key complexes have not been established for most tumors, our studies lay the groundwork and principles necessary for the optimal preclinical development and the choice of malignancy probably to be targeted by specific BH3-mimetics currently under development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Andreas Strasser, Jerry Adams, Peter Czabotar, John Parisot, and David Segal for useful discussions; Gordon Smyth for statistical advice; Abbott for providing ABT-737 and 263; Lorraine O'Reilly and Kylie Mason for reagents and antibodies; Bruno Helbert and Carley Young for mouse genotyping; and Emily Sutherland, Tania Camilleri, and Giovanni Siciliano for excellent mouse husbandry.
This work was supported by the Australian Research Council (D.M.), Australian National Health and Medical Research Council (NHMRC; program grants 461219 and 461221, project grant 637326), Research Fellowships (A.W.R. and D.C.S.H.) and Career Development Award (W.D.F. and P.B.), the Lady Tata Memorial Trust (S.G.), the Leukemia Foundation of Australia Fellowships (S.L.K. and E.F.L.), Cancer Council Victoria, the Leukemia & Lymphoma Society (SCOR grant 7413), the National Cancer Institute (CA43540), and infrastructure support from the NHMRC (IRISS), and the Victorian State Government (OIS).
National Institutes of Health
Authorship
Contribution: D.M. and S.L.K. conducted most of the experiments; S.P.G. generated the Mcl-1fl/fl/Eμ-Myc lymphoma cells; L.D.B. and P.Y. performed the microarray experiments; M.R. helped with FACS analysis; B.P. analysed results and the heat-maps; W.D.F. and E.F.L. did the Biacore measurements; K.J.C, C.J.V., and S.C. generated the vavP–Mcl-1 and vavP–Bcl-2/Bax- or Bak-deficient mice; C.H.W., D.J.A., and M.J.C.L. designed and performed the protein redistribution essays; A.W.R. gave useful advice; and D.M., S.L.K., D.C.S.H., and P.B. designed the project and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.J.C. is Beatson Institute for Cancer Research, Glasgow, United Kingdom.
Correspondence: Philippe Bouillet, The Walter and Eliza Hall Institute, 1G Royal Parade, Parkville, VIC 3052, Australia; e-mail: bouillet@wehi.edu.au.
References
Author notes
D.M. and S.L.K. contributed equally to this work.