In this issue of Blood, Rahmani et al show in preclinical studies that the combination of the multi-kinase inhibitor sorafenib with the BH3 mimetic obatoclax results in enhanced antileukemic effects compared with the effects of each agent alone.1 This work has important clinical implications because it describes a novel approach to overcome acute myeloid leukemia (AML) cell resistance by combining agents that are currently being investigated in trials as single agents.
Despite recent advances on our understanding of mechanism and cellular pathways that promote proliferation and survival of leukemia cells, there is a desperate need for the design and development of innovative therapeutic approaches for the treatment of patients with AML. Sorafenib is a multi-kinase inhibitor, which targets and inhibits several tyrosine and serine-threonine kinases, including FLT3, RAF, VEGFR, PDGF, and c-kit.2 This agent has shown substantial clinical efficacy and is currently approved by the US Food and Drug Administration for the treatment of advanced hepatocellular and renal cell carcinoma.2 There has been a particular interest in its potential use for the treatment of AML due to its ability to inhibit FLT3.2 FLT3 is mutated in approximately 30% of AML cases, with the most common mutation being the internal tandem duplication in the juxtramembrane domain.2 FLT3 mutations carry a poor prognosis and many of these patients respond poorly to standard chemotherapeutic regimens.2 Beyond sorafenib, there have been ongoing preclinical and clinical efforts using other specific kinase inhibitors, potentially more FLT3-specific than sorafenib.2 However, a strong interest in sorafenib remains because of its superior pharmacokinetics and pharmacodynamics.3 Sorafenib has a long plasma half-life, one of its major metabolites is more potent than the parent compound, and inhibits FLT3-ITD more effectively at plasma concentrations achievable in patients.3 In clinical trials, sorafenib has shown promising results either as a monotherapy or when combined with standard chemotherapy, with complete remissions (CRs) seen in some FLT3-ITD patients.4
Despite the promise of sorafenib as an agent with activity against FLT3-ITD, there are many potential limitations, including initial inherent resistance to FLT3 inhibitors due to concurrent downstream pathway activation, and emergence of mutations during therapy that reduce inhibition or prevent drug binding.2 Another important mechanism is the up-regulation of antiapoptotic Bcl-2 family members.5 Despite an intense focus on sorafenib in FLT3-mutated AML, one research group has previously shown that sorafenib, at clinically achievable concentrations, can kill wild-type FLT3 AML cells.6 Such antileukemic effects on FLT3 wild-type leukemia cells are characterized by down-regulation of the Mcl-1 protein through an effect on translation, suggesting an approach to circumvent resistance by targeting Mcl-1.6,7
Dysregulation of the Bcl-2 family of proteins leading to a resistance to chemotherapy has been well described in leukemia.8 The Bcl-2 family consists of both pro- and antiapoptotic family members.9 The antiapoptotic/prosurvival proteins are Bcl-2, Bcl-xL, Bcl-W, Mcl-1, and A1.9 They interact and suppress the activity of the proapoptotic proteins Bax and Bak.9 The BH3-only proteins Bim, Bid, Puma, Noxa, Bad, and Bik are capable of promoting apoptosis by binding to antiapoptotic proteins and preventing them from interacting with Bak and Bax.9 In the case of Bid and Bim, they can directly activate Bak and Bax.9 Because of the importance of these mechanisms in drug resistance, several compounds have been developed to directly target the antiapoptotic proteins. However, there has been evidence that AML cells develop resistance to ABT-737, a promising compound that inhibits Bcl-2/Bcl-xL/Bcl-W, because of up-regulation of Mcl-1, which ABT-737 does not target.10 For this reason obataclax is a superior compound especially in the setting of AML due to its ability to inhibit all the antiapoptotic proteins, including Mcl-1.11
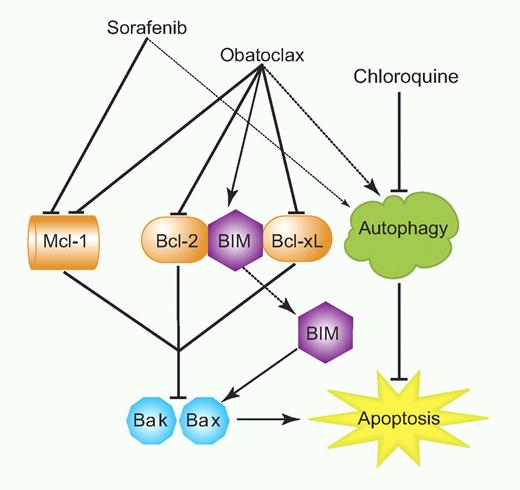
Here, Rahmani et al demonstrate enhanced apoptosis of AML cells treated with the kinase inhibitor sorafenib combined with the BH3 mimetic obatoclax. This is characterized by down-regulation of MCL-1, inhibition of BCL-2/BCL-xl, and enhanced activity of BIM and BAK (see figure). Importantly, such combined treatment specifically targets the AML cells directly while sparing normal CD34+ cells. Although these studies were focused mostly on AML cells expressing wild-type FLT3, the combined treatment did also show efficacy against cells from a patient expressing the FLT3− mutant as well. Thus, the combination of sorafenib and obatoclax may be effective in a wide range of AML patients and may be a combination clearly worth exploring in clinical trials. Rahmani and colleagues also demonstrate that knockdown of Bak and Bax abrogate some of the apoptotic effects of the 2 agents combined, suggesting that decreased expression or loss of either of these 2 proteins may result in leukemic cell resistance. However, obatoclax also causes cell-cycle arrest in addition to effects on apoptosis, indicating that even with the loss of Bak or Bax it may still have some antileukemic activity via other targets.11 Another important aspect of this study is the demonstration that induction of autophagy by the combination of sorafenib and obatoclax is cytoprotective. However, this can be overcome by inhibiting autophagy with chloroquine (see figure), raising the possibility that combined triple inhibition using sorafenib/obatoclax and an autophagy inhibitor may provide an approach to optimize efficacy.
Model for the enhancing effects of obatoclax on sorafenib-induced AML cell death. Professional illustration by Paulette Dennis.
Model for the enhancing effects of obatoclax on sorafenib-induced AML cell death. Professional illustration by Paulette Dennis.
Altogether, the results of this study are highly relevant and have important clinical-translational implications. Because of the efficacy of the combination of sorafenib and obataclax against AML cells, it would be interesting to examine in future studies whether this combination, alone or in combination with autophagy inhibitors, can eliminate leukemia-initiating cells (LICs). There are also other multi-kinase inhibitors that are either approved for treatment of specific solid tumors or are in clinical development. Combining such inhibitors with obatoclax may provide an approach to enhance their therapeutic efficacy in other malignancies beside AML. In a similar way, efforts to define the effects of combinations of obactolax with the next generation of FLT3 inhibitors are warranted and may provide an approach to enhance the antileukemic properties of such inhibitors as well.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health