In this issue of Blood, Juarez and colleagues reveal a need for the Sphingosine-1-Phosphate/S1P receptor 1 (S1P/S1P1) axis in the egress of hematopoietic stem cells/hematopoietic progenitor cells (HSCs/HPCs) from the bone marrow after CXCR4 antagonist mediated mobilization in mice.1 The authors also demonstrate an additional peripheral blood stem cell (PBSC) mobilization benefit for S1P1 agonist co-treatment in combination with a CXCR4 antagonist (see figure) but not human granulocyte colony-stimulating factor (G-CSF).
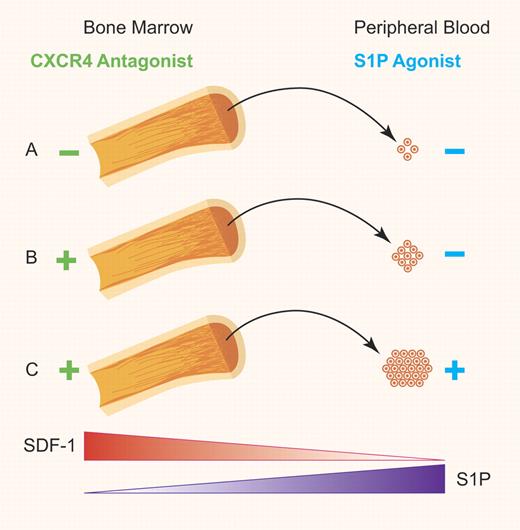
(A) Basal levels of HSC egress from the BM into the PB sinusoid. (B) Stimulated release of HSCs from the BM into the PB facilitated by the S1P/S1P1 axis gradient after disruption of the SDF-1/CXCR4 axis that normally retains HSCs in the bone marrow. (C) Increased levels of HSC mobilization into the PB achieved through augmentation of S1P in the PB in conjunction with disruption of SDF-1/CXCR4 axis. Professional illustration by Paulette Dennis.
(A) Basal levels of HSC egress from the BM into the PB sinusoid. (B) Stimulated release of HSCs from the BM into the PB facilitated by the S1P/S1P1 axis gradient after disruption of the SDF-1/CXCR4 axis that normally retains HSCs in the bone marrow. (C) Increased levels of HSC mobilization into the PB achieved through augmentation of S1P in the PB in conjunction with disruption of SDF-1/CXCR4 axis. Professional illustration by Paulette Dennis.
The observation that HPC numbers are elevated in the blood of patients after chemotherapy eventually led to the realization that HSCs/HPCs could be induced to exit the bone marrow and enter the circulation, a process referred to as mobilization. In the setting of hematopoietic stem cell transplantation (HSCT), patients are usually given chemotherapy, immunotherapy, and/or radiation therapy to treat their malignancy and then receive either their own autologous cells or allogeneic donor cells collected from a healthy individual. Currently, mobilized peripheral blood is the primary source of hematopoietic cells used for hematopoietic transplantation. This is particularly true in the adult transplantation setting where bone marrow (BM) harvest procedures have fallen out of favor, and where single umbilical cord blood (CB) collections are often too small in volume and/or cell number to be adequate for an adult recipient. While there is no universal consensus on optimal CD34+ cell yield after PBSC mobilization, infusions of greater than 2.0 to 2.5 × 106 cells/kg are generally associated with fast and sustained hematopoietic recovery. Larger doses of CD34+ cells may result in faster recovery after HSCT, and possibly higher overall recipient survival.2 Therefore, strategies to improve HSC/HPC yield during mobilization would have therapeutic benefit.
The ideal mobilization agent or regimen has yet to be identified, and numerous different mobilization approaches exist.3 Recombinant G-CSF is the most commonly used mobilizing agent in the clinical setting of allogeneic HSCT.4 Filgrastim (Neupogen; Amgen Inc), or methionyl human G-CSF (r-metHuG-CSF) is a G-CSF analog produced by recombinant DNA technology. It was initially used to treat chemotherapy-induced neutropenia, and is now known to increase the number of circulating HSCs. Mobilization with G-CSF alone is successful in approximately 85% of healthy volunteers.5,6 Additional mobilizing agents that have also been studied include recombinant human rhuGM-CSF (Sargramostim; Leukine, Bayer HealthCare Pharmaceuticals). However, because of an inferior CD34+ yield and marginal clinical benefits in this setting, its use as a mobilizing agent remains limited.3 Plerixafor (Mozobil; AMD3100, Genzyme Corp) is a bicyclam derivative that reversibly inhibits the binding of stromal cell–derived factor-1 (SDF-1/CXCL12), a chemokine, to its receptor, CXCR4.7 Plerixafor is thought to act as an antagonist (or possibly a partial agonist) of the α chemokine receptor CXCR4, and an allosteric agonist of CXCR7. In the autologous setting, the addition of Plerixafor to the G-CSF regimen for PBSC mobilization in patients with non-Hodgkin lymphoma (NHL) and multiple myeloma (MM) appears to increase CD34+ cell collection; resulting in fewer days of apheresis, and a higher proportion of patients proceeding to transplantation than with G-CSF alone. This suggests that regimens that mobilize PBSCs in donors who normally fail mobilization are of practical clinical benefit.
The role for the SDF-1/CXCR4 axis in retention of HSCs/HPCs in the BM under steady-state conditions is widely accepted. However, its role in stem cell trafficking, the processes of homing or mobilization, has needed additional clarification. At its most basic level, the question that persisted was; “If the SDF-1 gradient across the BM-PB barrier drives the homing of HSCs/HPCs into the BM during HSCT, what drives the release of cells from the BM microenvironment into the PB when the SDF-1/CXCR4 axis is disrupted?” Mechanistically, it appears that the homing of HSCs/HPCs from the PB into the BM is facilitated by the SDF-1/CXCR4 axis and that disruption of this axis results in the release of HSCs/HPCs. However, the model by which the SDF-1/CXCR4 axis changes during mobilization, thereby facilitating the release of HSCs/HPCs is unclear. It is in this context that the S1P active phospholipid has entered the discussion as a novel chemoattractant for HSCs/HPCs and a possible explanation for the mechanism behind release of HSCs/HPCs into PB. S1P, a product of 2 sphingosine kinases (SK1 and SK2), interacts with at least 5 G-protein–coupled seven-transmembrane–spanning receptors, S1P1-5, on the surface of target cells to induce cell migration. HSCs have been shown to express S1P receptors and migrate in response to S1P.8
Here, Juarez et al studied the role of the S1P/S1P1 axis in PBSC mobilization and determined that (1) pharmacologic inhibition of the S1P/S1P1 axis (using FTY720; Novartis) or use of S1P1-/- mice inhibits CXCR4 antagonist (AMD3100)–mediated mobilization but not G-CSF–induced mobilization, (2) use of SK1−/− mice inhibits AMD3100 mobilization, (3) S1P plasma levels increase after AMD3100 treatment in the PB of mice but not humans, and (4) treatment of mice with a S1P1 agonist (SEW2871) results in a dose-dependent augmentation of mobilization when co-administered with AMD3100. These data are consistent with other reports that suggest differing mechanisms of action between AMD3100 and G-CSF–induced mobilization of PBSCs. An unresolved question in the field is whether an increase in plasma S1P concentrations in the PB in response to stimulus is indeed a component of the mechanism by which PBSC mobilization occurs. Others have suggested that a S1P chemotactic gradient is continuously present in the PB, retention of HSCs/HPCs in BM is an active process that counteracts S1P gradient, and increased plasma S1P levels occurs during mobilization.9 The data presented here confirm that that is true in mice but will have to be examined in humans. Additional data in humans as well as mice using highly sensitive assays that are able to detect subtle changes in relatively low concentrations of S1P may help to resolve this question. Even with this remaining controversy it is clear that a combination strategy to disrupt the endogenous HSC/HPC retention mechanisms, such as the SDF-1/CXCR4 axis, while augmenting the S1P/S1P1 axis is likely to enhance PBSC mobilization and yield clinical benefit within the field of stem cell therapeutics/regenerative medicine.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■