Abstract
Although activity that induced tumor regression was observed and termed tumor necrosis factor (TNF) as early as the 1960s, the true identity of TNF was not clear until 1984, when Aggarwal and coworkers reported, for the first time, the isolation of 2 cytotoxic factors: one, derived from macrophages (molecular mass 17 kDa), was named TNF, and the second, derived from lymphocytes (20 kDa), was named lymphotoxin. Because the 2 cytotoxic factors exhibited 50% amino acid sequence homology and bound to the same receptor, they came to be called TNF-α and TNF-β. Identification of the protein sequences led to cloning of their cDNA. Based on sequence homology to TNF-α, now a total of 19 members of the TNF superfamily have been identified, along with 29 interacting receptors, and several molecules that interact with the cytoplasmic domain of these receptors. The roles of the TNF superfamily in inflammation, apoptosis, proliferation, invasion, angiogenesis, metastasis, and morphogenesis have been documented. Their roles in immunologic, cardiovascular, neurologic, pulmonary, and metabolic diseases are becoming apparent. TNF superfamily members are active targets for drug development, as indicated by the recent approval and expanding market of TNF blockers used to treat rheumatoid arthritis, psoriasis, Crohns disease, and osteoporosis, with a total market of more than US $20 billion. As we learn more about this family, more therapeutics will probably emerge. In this review, we summarize the initial discovery of TNF-α, and the insights gained regarding the roles of this molecule and its related family members in normal physiology and disease.
Introduction
The tumor necrosis factor (TNF) superfamily, composed of 19 ligands and 29 receptors, plays highly diversified roles in the body. The interest in TNF research has increased dramatically over the years as indicated by more than 113 000 citations on TNF-α alone, 27 000 on anti–TNF-α, 25 000 on TNF-α inhibitors, 55 000 on TNF receptors, 12 000 on TNF-mediated apoptosis, 40 000 on TNF-α-induced signals, and 9610 reviews. All members of the TNF superfamily, without exception, exhibit pro-inflammatory activity, in part through activation of the transcription factor NF-κB. Several members of the TNF superfamily exhibit proliferative activity on hematopoietic cells, in part through activation of various mitogen-activated kinases, and some members of this family play a role in apoptosis (Figure 1).1,2 Some members of the TNF superfamily have also been reported to play a role in morphogenetic changes and differentiation. Most members of the TNF superfamily have both beneficial and potentially harmful effects.3 Although TNF-α, for example, has been linked with physiologic proliferation and differentiation of B cells under steady-state conditions, it also has been linked with a wide variety of diseases, including cancer, cardiovascular, neurologic, pulmonary, autoimmune, and metabolic disorders.
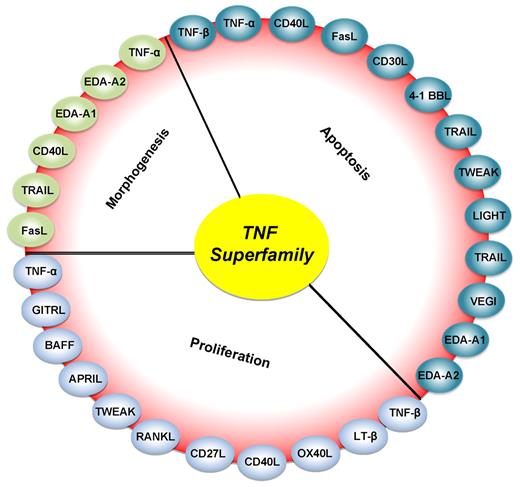
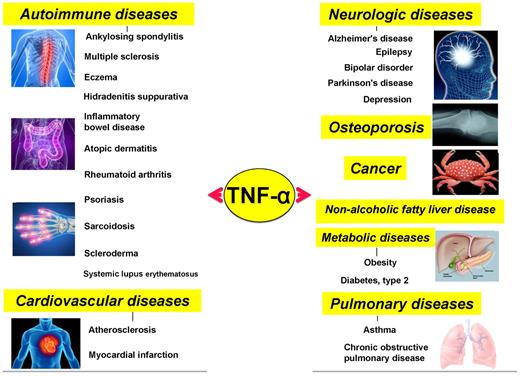
Roles of various members of the TNF superfamily in inflammation, cellular proliferation, apoptosis, and morphogenesis. All members of the TNF superfamily exhibit pro-inflammatory activity, in part through activation of the transcription factor NF-κB (full red circle); OX40L, CD40L, CD27L, APRIL, and BAFF exhibit proliferative activity in part through activation of various mitogen-activated kinases (sky blue); TNF-α, TNF-β, FasL, and TRAIL control apoptosis (bluish-green); and EDA-A1, EDA-A2, TNF-α, FasL, and TRAIL regulate morphogenesis (green).
Roles of various members of the TNF superfamily in inflammation, cellular proliferation, apoptosis, and morphogenesis. All members of the TNF superfamily exhibit pro-inflammatory activity, in part through activation of the transcription factor NF-κB (full red circle); OX40L, CD40L, CD27L, APRIL, and BAFF exhibit proliferative activity in part through activation of various mitogen-activated kinases (sky blue); TNF-α, TNF-β, FasL, and TRAIL control apoptosis (bluish-green); and EDA-A1, EDA-A2, TNF-α, FasL, and TRAIL regulate morphogenesis (green).
Although the fascinating history of TNF could be traced back more than one century, the name “tumor necrotizing factor” was first used in 1962 for tumor (sarcoma 37) regression activity induced in the serum of mice treated with Serratia marcescens polysaccharide; this activity was shown by Carswell et al in 1975 to be the result of TNF.4,5 Whether this TNF is the same as what our group later renamed TNF-α is unclear. In 1984 to 1985, our group structurally identified 2 different TNFs and cloned their genes. From hundreds of liters of conditioned medium collected from the human lymphoblastoid cell line RPMI 1788, we purified a protein 25 kDa in size, which initially was named lymphotoxin-α (LT-α) and later changed to TNF-β with the later discovery of sequence homology with TNF-α.6,7 Using the same cell lysis assays and antibodies against lymphotoxin, we then reported isolation of a second cytotoxic factor from hundreds of liters of conditioned supernatants of human promyelomonocytic cell line HL-60; this factor had a molecular mass approximately 17 kDa and was named human TNF-α.8 When we first performed the amino acid sequencing of the 2 proteins, it became clear that they exhibited as much as 50% sequence homology.6-8 Generation of antibodies against each molecule proved that these 2 proteins were immunologically distinct9 ; whereas TNF-α was produced by macrophages, TNF-β was produced by lymphocytes.10
The amino acid sequence was used to prepare and isolate the full-length cDNAs for TNF-α and TNF-β.11,12 The cloning of the cDNA for TNF-β turned out to be much more difficult than that of TNF-α, for unclear reasons; thus, for TNF-β, most of the gene actually had to be chemically synthesized on the basis of the protein sequence.12 Soon our group discovered that both TNF-α and TNF-β bind to a common high-affinity cell surface receptor present on most cell types.13 While we were finishing the isolation of the TNF-α and TNF-β from monocytes and lymphocytes, respectively, Beutler et al from Rockefeller University reported the identity of TNF with the macrophage-secreted factor cachectin,14 which was responsible for cachexia. This protein was found to be a murine counterpart of human TNF-α, based on the amino acid sequence homology to human TNF-α. Cerami gave his account of this event in a recent article.15 Although passive immunization against cachectin/TNF has been shown to protect mice from the lethal effect of endotoxin, there are reports suggesting that endotoxin-induced factor is distinct from TNF-α.14
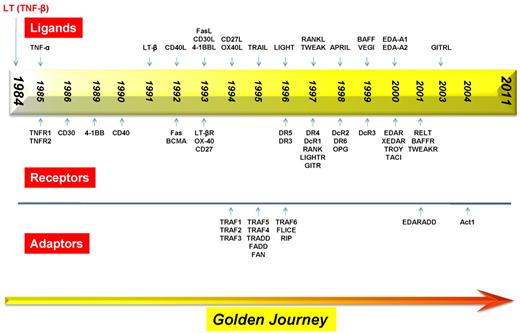
More than 25 years later, 19 different members of the TNF superfamily have been identified based on their gene sequences (Figure 2; Table 1). TNF-α and TNF-β are the only members of this family that were first identified at the protein level, before isolation of their cDNA; all other members of this family were identified via human cDNA sequence homology. The superfamily members are TNF-α, TNF-β, lymphotoxin-β, CD40L, FasL, CD30L, 4-1BBL, CD27L, OX40L, TNF-related apoptosis-inducing ligand (TRAIL), LIGHT, receptor activator of NF-κB ligand (RANKL), TNF-related weak inducer of apoptosis (TWEAK), a proliferation-inducing ligand (APRIL), B-cell activating factor (BAFF), vascular endothelial cell-growth inhibitor (VEGI), ectodysplasin A (EDA)–A1, EDA-A2, and GITRL. These 19 members bind to 29 different receptors (Figure 2; Table 1). In addition, 3 TNF receptors (ie, TNFRSF22-mDcTRAILR2/TNFRH2, TNFRSF23-mDcTRAILR1/TNFRH1, and TNFRSF26-mTNFRH3) have been identified in mice only.16 The TNF receptor NGFR differs in its biologic activity from other members. Furthermore, the nomenclature of the TNF superfamily ligands has been standardized by an international commission as TNF superfamily (TNFSF), and the receptors as TNFRSF (Table 1). The cell types expressing each ligand or receptor are well characterized and summarized in Table 1. Both the ligands and receptors are expressed primarily by various cells of the immune system, but other cell types have been shown to express ligands as well as the receptors, under both physiologic and pathologic conditions. The importance of these proteins/cytokines in the pathophysiology of human diseases is indicated by the fact that in 2010 sales of TNF blockers exceeded US $20 billion (www.biospace.com/News).
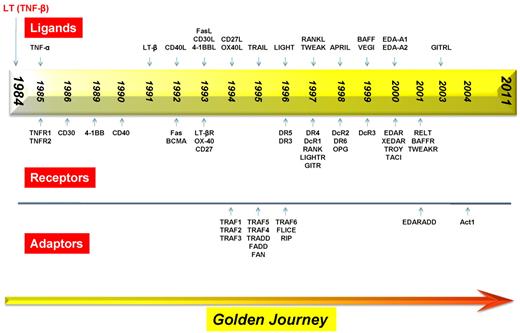
Timeline for the discovery of various members of the TNF superfamily, their receptors, and the receptor-associated adaptor proteins.
Timeline for the discovery of various members of the TNF superfamily, their receptors, and the receptor-associated adaptor proteins.
TNF-α was first identified as a factor with antitumor activity. The potent pro-inflammatory activity, however, prevents systemic administration of TNF-α to cancer patients.17,18 However, TNF-α is indeed used in the clinic. Specifically, it is used in the treatment of soft tissue sarcomas and melanomas in the extremities, using a technique called isolated limb perfusion. In this setting, TNF-α has demonstrated potent antitumor activity with an acceptable safety profile.19-21
In the sections to follow, we discuss the signaling events mediated by members of TNF superfamily and receptors with a particular emphasis on TNF-α. We describe the role of this superfamily in normal physiology and disease. Finally, we discuss rationally designed therapeutics that are based on TNF signaling.
Promiscuity and cell signaling of TNF superfamily
The mechanism by which TNF and its various family members transduce target cell signals has been studied extensively. That the 19 members of the TNF superfamily interact with 29 distinct receptors implies that at least some of the ligands must interact with more than one receptor. For instance, TNF-α is known to interact with 2 distinct receptors, TNF receptor 1 (TNFR1) and TNFR2 (Figure 3). On B cells, BAFF has been shown to bind to 3 distinct receptors, transmembrane activator and CAML interactor (TACI), B-cell maturation protein (BCMA), and BAFF receptor (BAFFR). TRAIL has been shown to bind to as many as 5 different receptors, including death receptor 4 (DR4), DR5, decoy receptor 1 (DcR1), DcR2, and osteoprotegerin (OPG).22 Whereas DR4 and DR5 transduce the signals across the cell membrane, DcR1 and DcR2 bind the ligand but do not transduce signals and OPG is primarily a soluble receptor without any transmembrane domain. Some of the cell-surface receptors bind more than one ligand, as in the case of TNF-α and TNF-β, both of which bind to the same receptor with comparable affinity.8
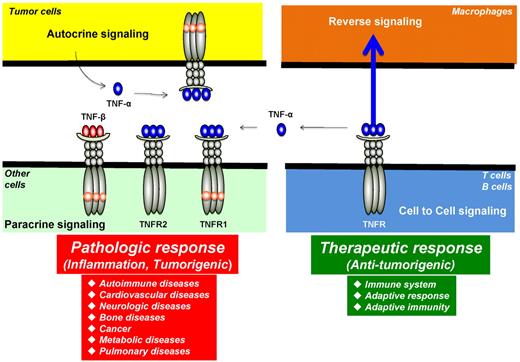
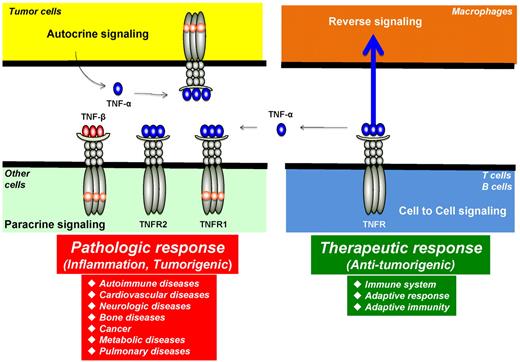
Autocrine, paracrine, cell to cell, and reverse signaling pathways for TNF-α. Ligands, such as TNF-α, are expressed as both transmembrane and in soluble forms. The transmembrane form of the ligand appears to mediate therapeutic effects, but soluble ligand is linked to pathologic effects of TNF-α. Ligands, such as TNF-β, lack the transmembrane domain and thus are expressed only as a soluble protein. TNF-α, made by tumor cells, acts primarily through TNFR1 in an autocrine and paracrine manner. There are also examples of reverse signaling through TNF-α when it binds to its receptor.
Autocrine, paracrine, cell to cell, and reverse signaling pathways for TNF-α. Ligands, such as TNF-α, are expressed as both transmembrane and in soluble forms. The transmembrane form of the ligand appears to mediate therapeutic effects, but soluble ligand is linked to pathologic effects of TNF-α. Ligands, such as TNF-β, lack the transmembrane domain and thus are expressed only as a soluble protein. TNF-α, made by tumor cells, acts primarily through TNFR1 in an autocrine and paracrine manner. There are also examples of reverse signaling through TNF-α when it binds to its receptor.
Most of the receptors of the TNF superfamily can be classified into one of 2 categories: those that possess an intracellular death domain (DD) and those that do not. The DD is a region approximately 45 amino acids long that is required for recruitment of other proteins resulting in cell death. Six different receptors have been identified with DD in their intracellular domain: DR1 (also called TNFR1); DR2 (also called Fas); DR3, to which VEGI binds; DR4 and DR5, to which TRAIL binds; and DR6. No ligand has yet been identified that binds to DR6. Interestingly, a recent study indicates that DR6 is broadly expressed by developing neurons and is activated by β-amyloid precursor protein.23 The authors suggested that an extracellular fragment of β-amyloid precursor protein, acting via DR6 and caspase-6, contributes to Alzheimer disease.
The expression of various receptors of the TNF superfamily may vary significantly between cell types and tissue. Receptors that contain a DD in their intracellular domain are expressed most universally. TNF receptor 1 (TNFR1), which contains a DD, is highly promiscuous and is expressed on every cell type in the body studied to date. This may reflect the receptor's diverse functions in different cell types. Similarly, DR4 and DR5 have been shown to be expressed in most cell types. The expression of TNFR2, in comparison, is limited to cells of the immune system, endothelial cells, and nerve cells. Fas receptor expression is also highly heterogeneous and is found on epithelial cells, hepatocytes, and lymphocytes.
Cell signaling for most cytokines and growth factors is normally mediated through interaction between a soluble ligand and a transmembrane receptor. Among the TNF superfamily, however, several ligands have been identified that rarely appear as soluble ligand, including FasL, CD27L, CD30L, CD40L, OX40L, and 4-1BBL. All of these ligands are instead primarily expressed as transmembrane proteins on the cell surface and interact with cells that express the corresponding receptors. Some ligands, such as TNF-α, are expressed as both transmembrane and soluble forms, whereas ligands, such as TNF-β, lack the transmembrane domain and thus are expressed only as a soluble protein. Obviously, cell signaling effects are more restricted when a ligand is ex-pressed only as a transmembrane protein. There are reports, furthermore, that the nature of cell signaling may differ between ligands expressed as soluble protein and those expressed in transmembrane form.3
Another characteristic feature of some ligand-receptor interactions in the TNF superfamily is the phenomenon of “reverse signaling”: instead of signaling being transmitted from ligand to receptor, it is transmitted from receptor to the cell bearing the transmembrane form of the ligand (Figure 3).24 For example, one report showed that TNF-α, when activated by TNF-α antibody, induced E-selectin (CD62E) expression on activated human CD4+ T cells via an outside-to-inside signal through the membrane.25 This report indicates that membrane TNF-α can transmit bipolar signals. Reverse signaling has also been reported via the CD30 ligand.26 CD30 ligand is a type II membrane protein with a C-terminal extracellular domain that is homologous with the extracellular domains of other TNF family members. Cross-linking of CD30 ligand by a monoclonal antibody or by CD30-Fc fusion protein induced production of IL-8 by freshly isolated neutrophils. CD30 ligand, but not CD30, is expressed on neutrophils. Clearly, several TNF family members and their cognate receptors signal bidirectionally, thus blurring the distinction between ligand and receptor. How this kind of signaling is mediated is not fully understood.
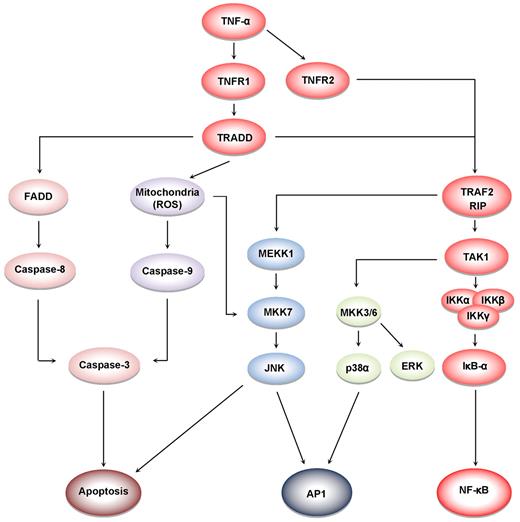
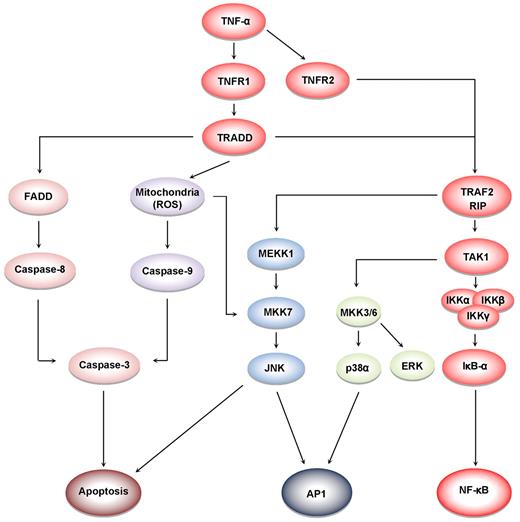
TNF-α induces at least 5 different types of signals that include activation of NF-κB, apoptosis pathways, extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (p38MAPK), and c-Jun N-terminal kinase (JNK; Figure 4). When TNF-α binds to TNFR1, it recruits a protein called TNFR-associated death domain (TRADD) through its DD.27 TRADD then recruits a protein called Fas-associated protein with death domain (FADD), which then sequentially activates caspase-8 and caspase-3, and thus apoptosis.28 Alternatively, TNF-α can activate mitochondria to sequentially release ROS, cytochrome C, and Bax, leading to activation of caspase-9 and caspase-3 and thus apoptosis.29 Paradoxically, TNF-α has also been shown to activate NF-κB, which in turn regulates the expression of proteins associated with cell survival and cell proliferation.30 NF-κB activation by TNF-α is mediated through sequential recruitment of TNFR1, TRADD, TNFR-associated factor 2 (TRAF2/TRAF5), receptor interacting protein (RIP), TGF-β–activated kinase 1 (TAK1), IκB kinase (IKK) complex, and inhibitor of nuclear factor-κBα (IκBα) phosphorylation, ubiquitination, and degradation, and finally nuclear translocation of p50 and p65 and DNA binding.31 The pro-inflammatory effect of TNF is mediated through NF-κB–regulated proteins, such as IL-6, IL-8, IL-18, chemokines, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and 5-lipoxygenase (5-LOX), all major mediators of inflammation. Indeed, TNF-α can induce expression of TNF-α itself through activation of NF-κB.3 TNF-α can also activate cellular proliferation through activation of another transcription factor, activator protein-1 (AP-1),32 which is activated by TNF-α through sequential recruitment of TNFR1, TRADD, TRAF2, MAP/ERK kinase kinase 1 (MEKK1), MAP kinase kinase 7 (MKK7), and JNK. The activation of p38MAPK by TNF-α is mediated through TRADD-TRAF2-MKK3. How TNFR2, which lacks a DD, activates cell signaling is much less clear than how TNFR1 activates cell signaling. Because TNFR2 can directly bind to TRAF2, it can activate both NF-κB and MAPK signaling quite well. Interestingly, TRADD has been reported recently to mediate cell signaling by TOLL-like receptors 3 and 4.33
Cell signaling pathways activated by TNF. TNFR1 activation leads to recruitment of intracellular adaptor proteins (TRADD, FADD, TRAF, and RIP), which activate multiple signal transduction pathways. TNFR sequentially recruits TRADD, TRAF2, RIP, TAK1, and IKK, leading to the activation of NF-κB31 ; and the recruitment of TRADD, FADD, and caspase-8, leads to the activation of caspase-3, which in turn induces apoptosis.28 JNK is activated through the sequential recruitment of TRAF2, RIP, MEKK1, and MKK7.79 Exposure of cells to TNFα in most cases results in the generation of reactive oxygen species, leading to activation of MKK7 and JNK.29 The activation of ERK and p38MAPK is via TRADD, TRAF2, RIP, TAK1, and MKK3/6.80
Cell signaling pathways activated by TNF. TNFR1 activation leads to recruitment of intracellular adaptor proteins (TRADD, FADD, TRAF, and RIP), which activate multiple signal transduction pathways. TNFR sequentially recruits TRADD, TRAF2, RIP, TAK1, and IKK, leading to the activation of NF-κB31 ; and the recruitment of TRADD, FADD, and caspase-8, leads to the activation of caspase-3, which in turn induces apoptosis.28 JNK is activated through the sequential recruitment of TRAF2, RIP, MEKK1, and MKK7.79 Exposure of cells to TNFα in most cases results in the generation of reactive oxygen species, leading to activation of MKK7 and JNK.29 The activation of ERK and p38MAPK is via TRADD, TRAF2, RIP, TAK1, and MKK3/6.80
Although members of the TNF superfamily have the potential to activate NF-κB, almost all of them, unlike TNF-α, activate NF-κB in a cell type-specific manner. This is restricted in part by the expression of cell surface receptors on particular cell types and in part through independent mechanisms yet to be determined. For instance, DR2, DR4, and DR5 are expressed on most cell types, but their interactions with respective ligands rarely lead to NF-κB activation. On the other hand, interaction of DR1 with its ligand leads to NF-κB activation in most cells. This ability of TNF-α to activate NF-κB nonselectively makes it the most promiscuous and universal pro-inflammatory family member in most situations. TNF-β has been shown to be a less potent pro-inflammatory agent than TNF-α.34
Although various members of the TNF superfamily are known to activate apoptosis, most cells are resistant to apoptosis induced by TNF-α alone.35 The mechanism for this resistance to apoptosis is not understood; it is not the result of lack of receptors. It seems that cell survival, proliferative, and apoptotic signals are all activated simultaneously by TNF-α and related proteins and that the balance between these signals determines whether the TNF family member induces apoptosis, proliferation, versus no effect at all. Whereas new protein synthesis is usually required for survival and proliferative signals, no protein synthesis is required for apoptosis.
Life with TNF-α
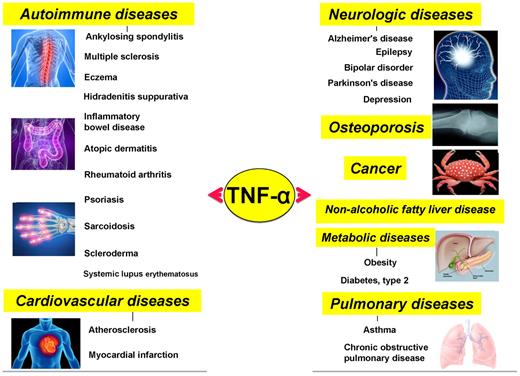
Although initially discovered as an anticancer agent, TNF-α and its family members have now been linked to an array of pathophysiologies, including cancer, neurologic diseases, cardiovascular diseases, pulmonary diseases, autoimmune diseases, and metabolic diseases.
TNF-α and cancer
The carcinogenic activities of TNF-α are mediated through its ability to activate the pro-inflammatory transcription factor NF-κB, which up-regulates the expression of genes linked to tumor cell survival, proliferation, invasion, angiogenesis, and metastasis.36 Several tumor cell types constitutively express TNF-α, including ovarian cancer, breast cancer, and others.37 Most tumor cells that express TNF-α exhibit constitutive activation of NF-κB. These tumors cells are “addicted” to NF-κB, as their survival is highly dependent on this factor.
In contrast to TNF-α, other members of the TNF superfamily exhibit more anticancer as opposed to oncogenic potential. For instance, TRAIL is being actively explored as an anticancer agent because of its preferential ability to induce apoptosis in tumorigenic or transformed cells, but not in normal cells or tissues.38 Like TNF-α, CD27L, CD30L, CD40L, APRIL, and BAFF, although essential regulators of the immune system, play a major role in hematopoietic tumorigenesis.
TNF-α and neurologic diseases
Both TNF-α and its receptors are expressed by microglial cells in the brain. Through activation of NF-κB, TNF-α plays an essential role in the survival of these cells. In the brain, TNF-α has been shown to induce pro-inflammatory signals that have been linked with depression,39 bipolar disporder,40 epilepsy,41 Alzheimer disease,42 Parkinson disease,43 and multiple sclerosis.44 Similarly, Fas ligation has been shown to induce selective expression of chemokines, IL-8, and monocyte chemoattractant protein-1 (MCP-1) in human astroglioma cells in vitro.45
TNF-α and cardiovascular diseases
TNF-α, along with other inflammatory molecules, is known to play a role in the initiation and progression of cardiovascular diseases. Although normal heart does not express TNF-α, the failing heart produces massive amounts of TNF-α. There is now growing evidence that the immune system is an important source for TNF production in failing heart. However, myocardium may also synthesize TNF-α de novo in failing heart.46 Perhaps the earliest indication that TNF-α is linked with heart failure emerged in 1990 when levels of circulating TNF-α levels were found to be elevated in sera of patients with chronic heart failure.47 Since then, numerous reports and reviews have been published on this subject.48 Evidence indicate that sustained and excessive production of TNF-α worsens the disease.49
Reports indicate that patients with chronic inflammatory disorders, such as rheumatoid arthritis (RA) and psoriasis, exhibit higher than expected rates of mortality that cannot be explained by traditional risk factors alone.50 This statement implies that inflammatory pathways might also contribute to the increased vascular risk in such diseases. The epidemiologic, physiologic, and model data suggest that TNF-α is directly involved in vascular pathophysiology. According to one published report, there are approximately 50% greater chances of cardiovascular mortality in RA patients compared with the general population.51
TNF-α and pulmonary diseases
TNF-α has been shown to play a major role in various pulmonary diseases, including asthma, chronic bronchitis, chronic obstructive pulmonary disease, acute lung injury, and acute respiratory distress syndrome.52 TNF-α is expressed in asthmatic airways and has been shown to play a role in amplifying asthmatic inflammation through the activation of NF-κB, AP-1, and other transcription factors.53 TNF-α has also been shown to induce pathologic features associated with COPD in animal models, such as an inflammatory cell infiltrate into the lungs, pulmonary fibrosis, and emphysema.54 TRAIL has also been linked to an inflammatory response in asthma.55 Interestingly, although TNF-α and TRAIL mediate lung inflammation, signaling through DR3 has been linked with expansion of Tregs, which plays a protective role against allergic lung inflammation in a mouse model of asthma.56
TNF-α, diabetes, and obesity
The first indication of increased cytokine release in obesity was provided by the identification of increased expression of TNF-α in the adipose tissue of obese mice in the early 1990s. TNF-α is expressed in and secreted by adipose tissue; its levels correlate with the degree of adiposity and the associated insulin resistance.57 The dephosphorylation of insulin receptor substrate-1 through activation of protein phosphatases has been shown to account for TNF-α-induced insulin resistance.57 Obesity is a leading cause of insulin resistance and type 2 diabetes, and targeting TNF-α and/or its receptors has been suggested as a promising treatment strategy for these conditions.58 Emerging evidence has indicated that inflammation is one of the critical processes associated with development of insulin resistance, diabetes, and related diseases, and obesity is now considered a state of chronic low-grade inflammation.59
TNF-α and autoimmune diseases
One area where TNF family members play a pivotal role is in autoimmune diseases. Uveitis, multiple sclerosis, systemic lupus, arthritis, psoriasis, and Crohn disease are all autoimmune diseases and are connected with dysregulation of various members of the TNF superfamily or their receptors as described here.60 Indeed, TNF-α blockers have been approved for the treatment of osteoarthritis, psoriasis, and Crohn disease. Among all the members of the TNF superfamily, the role of TNF-α in autoimmune diseases is best understood.
Life without TNF-α
From the studies already described, it is clear that both TNF-α and its family members play critical roles in the immune, cardiovascular, pulmonary, metabolic, and neurologic systems. Further insights into the function of these cytokines have been gained through gene deletion/gene knockout studies. The genes for the ligands and the receptors of the TNF superfamily have been deleted one by one in mice, and the resulting phenotypes have been examined (Table 2). Most commonly, defects in the immune system have been observed in these mice. For instance, deletion of TNF-β yielded a defect in development of secondary lymphoid organs and disorganized splenic architecture.61 Deletion of TNF-α, on the other hand, resulted in normal development and a complete lack of detectable morphologic or gross structural abnormalities.62 However, TNF-α-deficient mice were highly susceptible to challenge with an infectious agent and were resistant to the lethality of minute doses of lipopolysaccharide after D-galactosamine treatment.63 Amazingly, deletion of TNF-α had no effect on phagocytic activity of macrophages or on T-cell functions, as measured by proliferation, cytokine release, or cytotoxicity, suggesting redundancy with other superfamily members.
The deletion of the TRAIL gene has been shown to affect the retinal vascularization in mice.64 Generally, deletions of a ligand and its receptor have been shown to produce related phenotypes; however, in some cases, the 2 phenotypes are quite dissimilar. For instance, TRAIL is known to bind 2 different signaling receptors, DR4 and DR5, in humans but only DR5 in mice. Whereas deletion of TRAIL affected susceptibility to tumor burden and accelerated autoimmune diseases,65 animals with deletion of DR5 developed normally but had an enlarged thymus.66 This indicates that the ligand may also function independently of the receptor.
Mutation of TNF-α and its superfamily
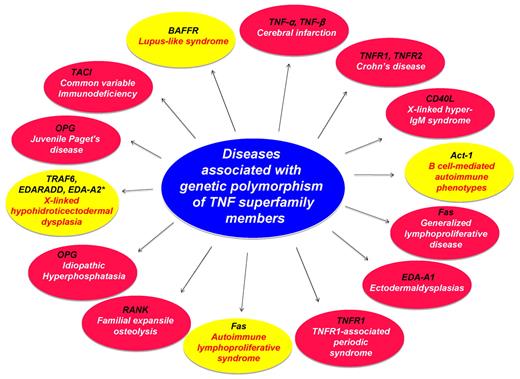
Various mutations in genes for TNF, its family members, and its receptors have been identified in humans (Table 3). These mutations are reflected either by a change in phenotype or a change in function. For instance, abnormalities in the CD40L gene have been linked to the X-linked immunodeficiency hyper-IgM syndrome. This human disease is characterized by elevated concentrations of serum IgM and decreased amounts of all other immunoglobulin isotypes. The CD40L protein produced in these patients was incapable of binding to CD40 and thus was unable to induce proliferation or IgE secretion from normal B cells. Activated T cells from some of these affected patients failed to express functional CD40L.
TNFR1-associated periodic syndrome (TRAPS) is the result of a mutation in the TNF receptor gene and is associated with systemic lupus erythematosus (SLE).67 Two biallelic polymorphisms in TNF-α (TNF-α–308) and TNF-β (TNF-β +252) genes have been associated with TNF production and susceptibility to inflammatory diseases.68 Two genetic polymorphisms in the TNF locus (TNF-α–308 G → A and TNF-β +252 A → G) were found to be risk factors for cerebral infarction.68 These mutations provide novel insights into the function of various members of the TNF superfamily.
TNF-α–based therapeutics
TNF superfamily members play a role in the pathogenesis of various human diseases (Figures 5 and 6). Thus, this superfamily represents an active target for drug development. Various antagonists against TNF family members, and their receptors have been approved by FDA and some are in clinical trials (Table 4). These agents are effective against RA, psoriatic arthritis, psoriasis, ulcerative colitis, and Crohn disease. Approved therapeutics are primarily one of 2 types of antagonist, either a soluble receptor that binds circulating TNF-α and acts as a “sink,” preventing interaction with cell surface receptors, or antibodies against TNF-α. Golimumab is a fully humanized TNF-α monoclonal antibody that is specific for human TNF-α and was approved for RA, psoriatic arthritis, and active ankylosing spondylitis.69 Similarly, Certolizumab pegol, a PEGylated Fab′ fragment of a humanized TNF antibody, is a monoclonal antibody directed against human TNF-α. Certolizumab pegol was approved for RA and Crohn disease.70 A human antibody against RANKL (denosumab, Prolia) was recently approved for the treatment of osteoporosis.
Various diseases that have been closely linked to TNF-α and members of its family.
Various diseases that have been closely linked to TNF-α and members of its family.
Diseases caused by mutation of genes in members of the TNF superfamily, its receptors, and adaptor proteins. Red circle represents studies in human; and yellow circle, studies in mice. Asterisk (*) within yellow circle indicates diseases in dogs.
Diseases caused by mutation of genes in members of the TNF superfamily, its receptors, and adaptor proteins. Red circle represents studies in human; and yellow circle, studies in mice. Asterisk (*) within yellow circle indicates diseases in dogs.
Several other products are currently in clinical trials. Atacicept is a human recombinant fusion protein designed to inhibit B cells, thereby suppressing autoimmune disease. This fusion protein composes the binding portion of a receptor for both BLyS and APRIL. Atacicept blocks activation of B cells by TACI, a transmembrane receptor protein found predominantly on the surface of B cells, and thus production of autoantibodies. The efficacy of atacicept in animal models of autoimmune disease and the biologic activity of atacicept in patients with SLE and RA has been demonstrated.71 Two recent phase 2 clinical trials evaluated the safety, efficacy, and biologic activity of atacicept in patients with active RA and an inadequate response to methotrexate. Despite the biologic effects of atacicept, the proportion of patients meeting the primary efficacy endpoint did not differ significantly in the atacicept groups and the placebo group.72,73 Another phase 2 clinical trial was designed to assess the safety and tolerability of atacicept and its effects on central nervous system inflammation in relapsing multiple sclerosis (IMP28063, www.clinicaltrials.gov, #NCT00642902). Surprisingly, an increase in inflammatory disease activity was reported that led to a suspension of all atacicept trials in multiple sclerosis.
One of the major problems with most of these TNF blockers is that they produce numerous side effects. For example, infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira) are immunosuppressants and carry black-box warning labels regarding the increased risk of serious infections (such as tuberculosis). These drugs also carry a warning label regarding an increased risk of central nervous system demyelinating disorders. Adverse reactions in patients receiving infliximab have been studied and reported. Risks include serious and sometimes fatal blood disorders, serious infections, lymphoma, and solid tumors. There are reports of serious liver injury, reactivation of hepatitis B, hepatosplenic T-cell lymphoma, and drug-induced lupus. Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia (some fatal) have also been reported with infliximab. Besides safety issues, most of the TNF blockers are highly expensive. For instance, infliximab can cost as much as $22 000 a year per patient. Thus, alternatives that are safer and more affordable, yet effective, are needed.
TNF-α and the golden spice
Attempts have been made over the past several years for the identification of agents that are safe, efficacious, and inexpensive. Agents derived from natural sources have gained considerable attention because of their ability to suppress TNF expression and TNF signaling. Curcumin (diferuloylmethane) is one such agent that was isolated more than a century ago from the rhizomes of the golden spice turmeric (Curcuma longa). While searching for a TNF blocker, our group was the first to demonstrate that curcumin can block the activity of TNF-α,74 and another group showed that it can also suppress TNF production.75 Since then, we and others have shown its ability to inhibit the growth of a wide variety of tumor cells through suppression of TNF signaling. Curcumin has also been shown to inactivate TNF-α by direct binding.76 In one study, curcumin docked at the receptor-binding sites of TNF-α and exhibited direct interaction by both noncovalent and covalent interactions.77 Curcumin is currently in clinical trials for all those diseases for which TNF blockers have been approved. In addition, several other natural products are also known to suppress both the production and action of various members of the TNF superfamily.78
Future of TNF research
From the studies described here, it is clear that TNF-α and its family members constitute a very active area of research and thus has opened up numerous possibilities for treatment of variety of human diseases, including osteoporosis, psoriasis, arthritis, and Crohn disease. The number of publications on this superfamily, their involvement in a wide variety of human diseases, their potential to treat human disease, and the interest in and sales of products based on this family all are indicators of the future potential of these agents. However, this research is just beginning and will probably continue for decades to come. It is becoming increasingly evident that, although under controlled circumstances acute inflammation has therapeutic potential, when out of control, chronic inflammation can mediate most chronic diseases. Members of the TNF superfamily are major mediators of chronic inflammation and thus need to be regulated. More understanding is required of the pathways activated by these ligands and how to interrupt these pathways selectively for the benefit of mankind.
In conclusion, the golden journey of TNF started almost 25 years ago with the discovery of the 2 proteins, discovery of their amino acid sequence, cloning of their genes, and identification of their receptors. A great deal has been learned about cell signaling by these proteins, the cellular responses, their physiologic and pathologic roles, up-regulation and down-regulation, and genetic alterations. Most of the information in hand is about TNF-α, however; and very little is known about TNF-β, which binds to the same receptor as TNF-α. For instance, is TNF-β as pro-inflammatory as TNF-α, and if not, why not? What is the true physiologic role of TRAIL? Is this cytokine designed to kill tumor cells or is its true role yet to be determined? What is the role of VEGI? How does it signal? What is the ligand for DR6 or DR3, and how do they signal? Almost 50 different proteins constitute ligands of the TNF superfamily, ligand receptors, and receptor-associated proteins. Much is yet to be learned about them, and we expect this information to be forthcoming.
Acknowledgments
The authors thank Kathryn Hale for carefully proofreading the manuscript and providing valuable comments.
This work was supported by the National Institutes of Health (Cancer Center Core grant CA-16672 and Program Project grant CA-124787-01A2) and the Center for Targeted Therapy at The University of Texas M. D. Anderson Cancer Center. B.B.A. is the Ransom Horne Jr Professor of Cancer Research.
Personal account of corresponding author: In May 1980, Dr Aggarwal left the Hormone Research Laboratory of the University of California, San Francisco, to join a start-up company in South San Francisco called Genentech. Soon after joining Genentech, he was approached by the founder and president of the company, the young entrepreneur Bob Swanson, who was obsessed with gene cloning. Bob walked into Dr Aggarwal's office and asked him to find a “cure for cancer.” When Dr Aggarwal asked how he was supposed to do that, the immediate answer of Bob was that lymphokines are a cure for cancer and if he could clone this “sucker” he'll have a cure. After extensive reading, attending a conference in Dallas, and discussions with Dr Gale Granger, a pioneer in the lymphotoxin field, Dr Aggarwal learned that lymphokines are nothing but superatants from activated lymphocytes, and were being used at the time for the treatment of cancer. With this background, Dr Aggarwal got started in an area he had known nothing about.
National Institutes of Health
Authorship
Contribution: B.B.A. and J.H.K. reviewed the literature; J.H.K. wrote portions of the tables and figures; B.B.A. and S.C.G. wrote portions of the manuscript; and B.B.A. had overall editorial responsibility for the manuscript, tables, and figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bharat B. Aggarwal, Department of Experimental Therapeutics, The University of Texas M. D. Anderson Cancer Center, 1901 East Rd, Unit 1950, Houston, TX 77054; e-mail: aggarwal@mdanderson.org.