Abstract
Current factor IX (FIX) products display a half-life (t1/2) of ∼ 18 hours, requiring frequent intravenous infusions for prophylaxis and treatment in patients with hemophilia B. This open-label, dose-escalation trial in previously treated adult subjects with hemophilia B examined the safety and pharmacokinetics of rFIXFc. rFIXFc is a recombinant fusion protein composed of FIX and the Fc domain of human IgG1, to extend circulating time. Fourteen subjects received a single dose of rFIXFc; 1 subject each received 1, 5, 12.5, or 25 IU/kg, and 5 subjects each received 50 or 100 IU/kg. rFIXFc was well tolerated, and most adverse events were mild or moderate in intensity. No inhibitors were detected in any subject. Dose-proportional increases in rFIXFc activity and Ag exposure were observed. With baseline subtraction, mean activity terminal t1/2 and mean residence time for rFIXFc were 56.7 and 71.8 hours, respectively. This is ∼ 3-fold longer than that reported for current rFIX products. The incremental recovery of rFIXFc was 0.93 IU/dL per IU/kg, similar to plasma-derived FIX. These results show that rFIXFc may offer a viable therapeutic approach to achieve prolonged hemostatic protection and less frequent dosing in patients with hemophilia B. The trial was registered at www.clinicaltrials.gov as NCT00716716.
Introduction
Hereditary deficiency of clotting factor IX (FIX), hemophilia B, results in spontaneous or traumatic bleeding into the joints, soft tissues, and body cavities.1 When not treated adequately with FIX replacement therapy, bleeding results in disability and increased risk of death.2 Early individualized prophylactic treatment can improve outcomes by reducing the incidence of hemarthroses and subsequent arthropathy.3-5
FIX replacement therapy has been used for ∼ 40 years, initially purified from plasma (pdFIX), and more recently manufactured as a recombinant human FIX (rFIX). Both pdFIX and rFIX achieve effective and safe hemostasis in the prophylactic and surgical settings.1 The current prophylaxis regimen is given 2-3 times weekly by intravenous infusion. The frequency is determined by the half-life (t1/2; ∼ 18 hours) and recovery of rFIX. The requirement for frequent dosing can be a deterrent for adhering to a prophylactic regimen because of concerns about venous access and associated complications, such as thrombosis and infection.6,7 Thus, extending rFIX t1/2 and prolonging its protective hemostatic effect8-10 may reduce the number of injections needed to achieve effective hemostasis with the use of a prophylaxis regimen or to control breakthrough bleeds in on-demand therapy.
rFIXFc is a recombinant monomeric fusion protein composed of a single molecule of FIX covalently fused to the human IgG1 Fc domain, showing increased circulating t1/2 and bleeding control in several species.10 Activity was achieved with no cleavable linker between FIX and Fc, in contrast to other fusion proteins.9 The Fc domain permits binding to the neonatal Fc receptor (FcRn), expressed widely within endothelial cells and other cell types, and also represents a natural molecule with no known inherent toxicity.10 FcRn is constitutively expressed throughout life and is responsible for protecting IgG1 and Fc-fusion proteins from lysosomal degradation.11,12 Fc-fusion proteins taken up by pinocytosis and/or endocytosis interact with FcRn, resident within endosomes, which, in turn, direct the Fc-fusion proteins back to the plasma membrane, reintroducing them into circulation in a pH-dependent manner.13 This recycling approach has been used to extend the t1/2 of Fc fusion–based drugs used clinically (eg, etanercept, romiplostim) and in development.14,15
In mice, rats, dogs, and nonhuman primates, the t1/2 of rFIXFc is 3- to 5-fold longer than rFIX,10 indicating for the first time that FIX is cleared, at least in part, via lysosomal degradation and that FIX t1/2 can be extended via redirection to a natural protective pathway mediated by FcRn. This is a first-in-human clinical study that investigates the safety and pharmacokinetics (PK) of a long-lasting FIX Fc-fusion protein in patients with hemophilia B.
Methods
Study design
This study was performed in accordance with the US Code of Federal Regulations and International Conference on Harmonisation Guidelines on Good Clinical Practices. Before any testing, approval from participating institutional review boards and written informed consents from all subjects were obtained in accordance with the Declaration of Helsinki.
This was a phase 1/2a, open-label, multicenter, dose-escalation study of single-dose rFIXFc in previously treated subjects with severe hemophilia B (clinicaltrials.gov identifier NCT00716716). The primary objective was to assess the safety of rFIXFc over 30 days, and the secondary objective was to estimate the PK parameters of rFIXFc at doses ranging from 12.5 to 100 IU/kg.
Subject eligibility was determined, and 1 dose of rFIXFc was infused intravenously over ∼ 10 minutes at 6 sequential dose levels: 1, 5, 12.5, 25, 50, and 100 IU/kg. Plasma samples to measure FIX activity at doses of ≥ 12.5 IU/kg were taken at baseline before infusion, immediately after infusion, and 0.25, 1, 3, 6, 9, 24, 48, 72, 96, 120, 168, and 240 hours (10 days) after infusion. At the 100-IU/kg dose, samples were also taken at 12 and 14 days.
Subjects
Male subjects ≥ 18 years of age with severe (defined as ≤ 2 IU/dL FIX:C) hemophilia B and ≥ 150 prior documented exposure days to other FIX products were included. Persons with a history of inhibitors, allergic, or anaphylactoid reactions associated with FIX or intravenous immunoglobulin, concurrent autoimmune disease, coagulation disorder other than hemophilia B, or who were taking medications that could affect hemostasis were excluded. For subjects with unknown genotypes, genotyping was performed (Mayo Clinic and Puget Sound Blood Center).
Treatment product
rFIXFc was produced in human embryonic kidney 293 cells extensively tested for stability, sterility, and viral contamination to ensure safety. The purified drug product is composed of a monomeric rFIX covalently fused through its carboxy terminus to the N-terminus of a Fc monomer, which forms a disulfide bond with a second Fc monomer during synthesis and secretion from the cells.10,15 rFIXFc was supplied as a frozen (−70°C) liquid containing 1000 IU of rFIXFc per 5 mL (200 IU/mL) of formulation buffer (10mM sodium phosphate buffer [pH 7.0], 145mM sodium chloride, and 0.1% polysorbate 20).
Outcome measures
Safety was evaluated by physical examination, vital signs, electrocardiogram, development of adverse events (AEs), neutralizing and total binding Ab development, and laboratory changes over time. Secondary end points included estimated PK parameters. Laboratory assessments included viral status, levels of D-dimer and thrombin-antithrombin (TAT) complex, hematology, serum chemistry, and urinalysis. FIX Ag and rFIXFc concentrations in plasma were measured by ELISAs specific for FIX or rFIXFc (lower limit of quantification [LLOQ] of 10 and 16 ng/mL, respectively). FIX activity was determined by the one-stage (activated partial thromboplastin time; aPTT) clotting assay with the use of Normal Reference Plasma as a calibrator (LLOQ, 1 IU/dL), which has a potency assigned against the World Health Organization 3rd international standard for factors II, VII, IX, and X. FIX inhibitor was measured by Nijmegen-modified Bethesda assay (LLOQ, 0.6 BU/mL), and the anti-FIXFc Abs were evaluated by a specific bridging electrochemiluminescent immunoassay (sensitivity, 250 ng/mL). Electrocardiograms were performed throughout the study and assessed for QT interval.
PK analyses
FIX activity PK parameters were determined for the 25-, 50-, and 100-IU/kg dose groups (n = 11). A baseline subtraction method was used to account for endogenous FIX and incomplete washout of the previous prophylactic dose when calculating the rFIXFc activity-versus-time profile. Baseline was defined according to the subjects' lowest FIX activity (at screening, before dose, or after dose) and FIX Ag levels prior to dosing. Six of 11 subjects had baseline activity ≤ 1%, and the remaining 5 subjects had baseline activities of 2%. If residual drug was present before dose, it was decayed with ordinary first-order elimination with a t1/2 that was based on that reported for BeneFIX (18 hours).16
The FIX activity-versus-time data were computer-fitted to a 2-compartment model in WinNonlin, version 5.2.1 (Pharsight Inc) for calculation of PK parameters, including maximum activity (Cmax), t1/2, clearance (CL), volume of distribution at steady state (Vss), area under the curve (time zero extrapolated to infinity; AUCINF), mean residence time (MRT), and incremental recovery (K). In addition, plasma FIX activity above the subject's baseline at 168 hours (7 days) after dosing and time after dosing when FIX activity declined to 3 and 1 IU/dL above the subject's baseline level were also estimated. Actual sampling times, doses, and infusion durations were used in all calculations.
Similar PK analyses were also applied to the rFIXFc Ag concentration-versus-time data. Because the sensitivity of the rFIXFc Ag ELISA was ∼ 10-fold greater than that of the FIX 1-stage (aPTT) clotting assay, Ag data from all subjects in the dose groups of 12.5-100 IU/kg up to 336 hours after dosing were included in the PK analysis. For derivation of rFIXFc Ag PK parameters, doses were converted to “mg/kg” according to rFIXFc-specific activity. The assay was specific for rFIXFc Ag; thus, the pretreatment baseline was below the limit of detection, irrespective of whether the subjects had circulating dysfunctional endogenous FIX Ag or residual exogenous FIX from their last infusion.
To construct the activity-time profiles at steady state that followed different dosing regimens, Monte Carlo simulation was conducted with the population PK model of rFIXFc. The mean estimates of model parameters (CL, volume of distribution, intercompartmental CL, and volume of the second compartment) in the tested population, the interindividual variance, and the residual variability were adopted from this phase1/2a study. One thousand subjects were simulated per dosing regimen for a total of 5 dosing cycles for each subject. The body weight (BW) was generated according to the published method,17 that is, based on a power equation of Z = BW−0.5. The median BW in 1000 subjects was assumed to be 75 kg. On the basis of the simulated activity-time profiles, the mean and 95% CI of the model-simulated drug activity-time profiles of the 1000 subjects was constructed graphically for different dosing regimens.
Statistical analyses
Summary descriptive statistics were presented for all safety and PK parameters by dose level. The correlations between the K value, CL, and Vss of rFIXFc activity with body weight, age, and endogenous FIX Ag levels were determined by linear regression with associated R2 and P value, using GraphPad Prism Version 5 (GraphPad Software Inc).
Results
Subject disposition
The study enrolled 15 subjects; 14 received an infusion of rFIXFc (Table 1). One subject failed to return for study drug infusion and was withdrawn from the study. One subject each received 1, 5, 12.5, or 25 IU/kg, and 5 subjects each received either 50 or 100 IU/kg. All 14 subjects were included in the safety analysis; 12 subjects, who received 12.5-100 IU/kg doses of rFIXFc, were included in the PK analyses. The activity PK results for one subject (12.5 IU/kg) were excluded because of insufficient evaluable data to estimate the elimination phase. No clinically relevant differences were observed among treatment groups. Subjects with a variety of hemophilia B genotypes, such as stop codon/nonsense and missense mutations, were included (Table 2). Several subjects had near absence of FIX Ag that correlated with markedly reduced FIX activity, whereas others with missense genotypes had more Ag than activity, indicating a dysfunctional circulating protein. The pretreatment FIX activity in 2 subjects exceeded 2 IU/dL, probably because of an incomplete washout from their last infusion of FIX concentrate on the basis of historical testing and disease phenotype.
Safety
A total of 16 AEs were reported by 7 subjects; AEs were distributed evenly across treatment groups (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Most AEs were mild or moderate; the 2 treatment-related AEs included dysgeusia and headache, which occurred in one subject in the 50-IU/kg group. Two subjects experienced serious AEs that required hospitalization (abdominal adhesions and depression); neither was considered related to the study drug. No clinically relevant changes occurred in laboratory values, QT interval, or vital signs. No allergic reactions were detected. All plasma samples tested negative for FIX inhibitors and anti-rFIXFc Abs.
Six subjects experienced bleeding episodes between 9 and 28 days after dosing, when infused rFIXFc had been washed out. There were no reports of thrombosis during the study. In laboratory testing of thrombogenic markers, 2 subjects experienced sporadic, variable increases in TAT complex (5.8-43.1 ng/mL) during frequent blood draws over the first 24-48 hours; increases were not correlated with FIX activity levels. The maximal TAT elevations were 4 and 8-fold above the upper limit of normal and were not correlated with D dimer levels that remained normal or with consistent patterns after dosing in both patients. The short t1/2 of TAT (15 minutes) suggested slight coagulation activation possibly because of documented difficult phlebotomies, because D-dimer formation (24 hour t1/2) remained negative.
Pharmacokinetics
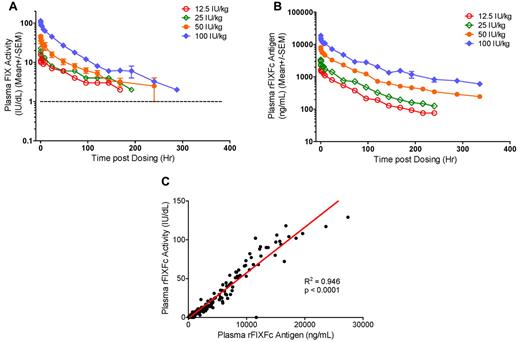
A dose-proportional increase in FIX activity was observed on the basis of Cmax occurring immediately after infusion and AUCINF (Table 3). FIX activity exhibited biexponential decay after infusion of rFIXFc and was characterized by a short distribution (α) phase followed by a log-linear elimination (β) phase (Figure 1A). The mean distribution t1/2 (t1/2α) was variable for individual subjects (mean, 3.31 and 10.3 hours for the 2 higher dose groups; Table 3). The mean elimination t1/2 (t1/2β) was dose independent over the therapeutic dose range tested, that is, 53.5 hours, 57.6 ± 8.27 hours, and 56.5 ± 14.1 hours at 25 IU/kg, 50 IU/kg, and 100 IU/kg, respectively. The mean t1/2β reported for rFIX (BeneFIX) is 19.3 ± 4.97 hours (range, 11.1-36.4 hours).16 The average time to 1% (1 IU/dL) above baseline, a surrogate assessment of rFIXFc efficacy, showed a dose-proportional increase. It was 7.34, 10.1 ± 1.58, and 12.3 ± 2.49 days for doses of 25, 50, and 100 IU/kg, respectively. The average time to 3% above base-line was 3.81, 6.28 ± 1.11, and 8.53 ± 1.58 days after doses of 25, 50, and 100 IU/kg, respectively. At 168 hours (1 week) after dosing, the plasma FIX activity was sustained at an average of 1.11 IU/dL, 2.47 ± 0.911 IU/dL, and 4.65 ± 1.73 IU/dL above baseline for the 25-, 50-, and 100-IU/kg dose groups, respectively. MRT, CL, and Vss were all dose independent. The mean MRT for all dose groups was 71.9 ± 9.66 hours (range, 53.2-85.9 hours), whereas the corresponding value reported for rFIX was 26.0 ± 6.07 hours (range, 15.8-46.1 hours). The mean CL of rFIXFc was 3.18 mL/h/kg, whereas the reported value for rFIX was 8.40 ± 2.01 mL/h/kg, and the mean Vss of rFIXFc was 227 ± 57.1 mL/kg (range, 162-296 mL/kg).16 Furthermore, each 1 IU/kg infused rFIXFc raised plasma FIX activity by 0.930 ± 0.179 IU/dL on average (Table 3), and this K showed weak positive correlation with BW (R2 = 0.336, P = .048; supplemental Figure 1). The corresponding values reported for rFIX were 0.75 ± 0.23 IU/dL per IU/kg (range, 0.34-1.38 IU/dL per IU/kg).16
Dose-dependent PK profiles and correlation of rFIXFc activity and Ag in plasma. (A) Plasma FIX activity and (B) plasma rFIXFc Ag levels over time after a single intravenous infusion of 12.5 (n = 1), 25 (n = 1), 50 (n = 5), or 100 (n = 5) IU/kg rFIXFc. Results presented are group mean ± SEM. (C) Correlation between plasma rFIXFc activity and Ag levels in 12 subjects who received a single dose of 12.5-100 IU/kg rFIXFc. Samples were collected up to 336 hours after dosing.
Dose-dependent PK profiles and correlation of rFIXFc activity and Ag in plasma. (A) Plasma FIX activity and (B) plasma rFIXFc Ag levels over time after a single intravenous infusion of 12.5 (n = 1), 25 (n = 1), 50 (n = 5), or 100 (n = 5) IU/kg rFIXFc. Results presented are group mean ± SEM. (C) Correlation between plasma rFIXFc activity and Ag levels in 12 subjects who received a single dose of 12.5-100 IU/kg rFIXFc. Samples were collected up to 336 hours after dosing.
Plasma rFIXFc Ag levels were measured by a rFIXFc-specific ELISA, and the concentration-versus-time curves are shown in Figure 1B. Findings from rFIXFc activity PK were corroborated by rFIXFc Ag PK (Table 4). Plasma rFIXFc concentrations also declined biexponentially after infusion, with the Cmax detected immediately after dosing. The plasma rFIXFc activity, as measured by the one-stage (aPTT) clotting assay, correlated well with rFIXFc Ag by ELISA in individual subjects (data not shown) and all subjects as a group (R2 = 0.946, P < .0001; Figure 1C). However, the mean dose-independent t1/2α (13.2 ± 3.95 hours), t1/2β (101 ± 20.9 hours), and MRT (110 ± 18.5 hours) of rFIXFc Ag were longer than those determined by plasma FIX activity measurements (Table 4).
Discussion
Studies have reported clear medical benefits with early individualized prophylactic therapy in hemophilia.20-22 To achieve wider acceptance, prophylaxis therapy needs to be effective, convenient, simple, and safe. Introduction of rFIXFc replacement therapy with a prolonged t1/2 may represent an important step toward achieving these goals.23 Moreover, a FIX product with an extended half-life compared with currently available products may have a greater effect in the treatment of episodic bleeds through a potential reduction in the number of follow-up treatments needed to control a bleed. A long-lasting FIX product may also influence defective wound healing, as has been described for hemophilia B.24
rFIXFc was not associated with inhibitor formation in any subject, all of whom had prior exposure to FIX products. Moreover, there was no evidence of allergic reactions or thrombogenicity as reported previously for rFIX.25 Levels of activated FIX are extremely low in rFIXFc, < 10% of the levels reported for currently marketed rFIX and 5% of the levels reported for pdFIX products.10
Results provide insight into the PK of rFIXFc in patients with hemophilia B. Cmax was reached immediately after infusion, suggesting a rapid onset of action similar to rFIX. The K observed for rFIXFc may represent an improvement compared with that reported for rFIX in 56 previously treated subjects.16 The improved recovery may be attributable to differences in posttranslational modification(s) from the human cell line, or the Fc moiety. In this study, the K of rFIXFc is weakly correlated with body weight (R2 = 0.336, P = .048), consistent with earlier reports,26-28 but no correlation was observed with age (R2 = 0.2783, P = .078) or endogenous FIX Ag level (R2 = 0.0023, P = .881; data not shown).
rFIXFc had a mean t1/2β and MRT ∼ 3-fold longer than that reported for rFIX.16,25 Furthermore, the ranges for rFIXFc and rFIX do not overlap and are consistent with the extended PK for rFIXFc observed in 4 different animal species.10 The t1/2α distribution phase averages ∼ 7 hours in patients who took rFIXFc, considerably longer than historical values for rFIX (2-3 hours). Similarly, the mean CL of rFIXFc activity is ∼ 2.6-fold less than that reported for rFIX.16
Although the same trend in improvement was observed in the rFIXFc Ag PK, both the t1/2α and t1/2β of rFIXFc Ag were longer than those derived from FIX activity. This discrepancy could in part be attributed to the markedly more sensitive Ag assay that enabled inclusion of Ag values detectable at much later time points (≤ 336 hours) in the calculation of the t1/2β. If the terminal Ag half-life is terminated at the same time points as the lowest reliably detected activity, the half-lives are similar at 73.0 ± 19.7 hours. Further studies in larger number of subjects will be important to assess rFIXFc activity versus Ag levels over time.
Data from PK analyses provide a means of optimizing individualized prophylactic treatment to achieve target trough levels > 1% (1 IU/dL) of baseline and to reduce peak/trough variation.29,30 In comparison with the recommended dose regimen of 25-40 IU/kg FIX twice weekly, the rFIXFc activity PK modeling results from this study suggest that once weekly dosing of rFIXFc at 20 IU/kg, or every 10 days at 40 IU/kg, or every 2 weeks at 100 IU/kg is sufficient to maintain a mean trough of 1% above baseline in the adult patient population with hemophilia B (Figure 2). These model-simulated estimates, however, must consider the heterogeneity of reported clinical breakthrough bleeding events relative to trough level of plasma FIX activity,31,32 in addition to the heterogeneity in PK parameters of individual subjects. Thus, prophylaxis dosing may require individual adjustment, as is often required now.
Monte Carlo simulation for rFIXFc doses to achieve a trough of 1 IU/dL (1%) or 3 IU/dL (3%) above baseline. The rFIXFc dosing intervals considered were (A) weekly, (B) every 10 days, or (C) every 2 weeks. The mean population PK parameters and relevant intersubject and intrasubject variability were adopted from this phase1/2a study. On the basis of the simulated activity-time profiles, the mean and 95% CI of the activity-time profiles of the 1000 subjects was constructed graphically for different dosing regimens for a total of 5 dosing cycles when the steady state was achieved.
Monte Carlo simulation for rFIXFc doses to achieve a trough of 1 IU/dL (1%) or 3 IU/dL (3%) above baseline. The rFIXFc dosing intervals considered were (A) weekly, (B) every 10 days, or (C) every 2 weeks. The mean population PK parameters and relevant intersubject and intrasubject variability were adopted from this phase1/2a study. On the basis of the simulated activity-time profiles, the mean and 95% CI of the activity-time profiles of the 1000 subjects was constructed graphically for different dosing regimens for a total of 5 dosing cycles when the steady state was achieved.
This study shows the safety and prolonged t1/2 of a novel therapeutic rFIXFc, resulting in rapid and prolonged circulating FIX activity. A larger clinical study to extend these observations is currently under way.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the study coordinators who made this trial possible: Angie Summers, LPN (Indiana Hemophilia and Thrombosis Center); Kristen Jaworski, RN (University of Pittsburgh); Elizabeth Jaksy, RN (Rush University Medical Center); Aime Grimsley, RN (University of North Carolina, Chapel Hill); Mark Bray (Puget Sound Blood Center); Janet A. Harrison (University of California–Davis); and Licca Yeh, RN, and Kenix Liu (Chinese University of Hong Kong). They also appreciate the work of Biogen Idec Hemophilia, the Biogen Idec and Swedish Orphan Biovitrum manufacturing groups, the bioanalytical assay groups at Biogen Idec and Biogen Idec Hemophilia (George Kamphaus, Nancy Moore, Tatiana Plavina, Donald Bennett, and Tamera Ashworth), the global clinical operations group at Biogen Idec and Biogen Idec Hemophilia (Ann Marie Mikols, Boyd Hanson, Byron McKinney, Jonathon Wiggins, Missy Magill, and Jocelyn Leone), Mark Rogge (Biogen Idec), and Seventh Wave Laboratories LLC for assistance with the pharmacokinetic analysis. They appreciate the helpful advice from Rick Blumberg, Professor of Medicine (Brigham and Women's Hospital) and Matt Ottmer and Samantha Truex (Biogen Idec Hemophilia). Editorial assistance was provided by Christine Kochi and Judy Berlfein at Biogen Idec Hemophilia. This study would not have been possible without the support of the patients who participated.
This work was supported in part by the National Center of Research Resources, National Institutes of Health (grant UL1RR025747) and in part by Swedish Orphan Biovitrum (SOBI).
National Institutes of Health
Authorship
Contribution: A.D.S. and M.V.R. conducted the research, analyzed the data, and wrote the manuscript; L.A.V., N.S.K., N.C.J., J.S.P., G.C., K.L.T., R.T.P., D.E., and A.J.B. performed research; A.R.T. performed genotype/phenotype analyses; and J.G., J.A.D., L.L., B.H., P.G., H.J. A.L., and G.F.P. designed the research, generated and analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: J.G., K.L.T, R.T.P., J.A.D., D.E., L.L., A.J.B., H.J., A.L., and G.F.P. are employees of Biogen Idec. B.H. and P.G. are employees of Swedish Orphan Biovitrum AB (publ). A.D.S., M.V.R., L.A.V., N.S.K., N.C.J., J.S.P., and G.C. received research support from Biogen Idec, which conducted the study. A.R.T. was a member of the external drug safety monitoring committee for this study.
Correspondence: Glenn F. Pierce, Biogen Idec Hemophilia, 133 Boston Post Rd, Weston, MA 02493; e-mail: glenn.pierce@biogenidec.com; and Amy Shapiro, Indiana Hemophilia and Thrombosis Center, 8402 Harcourt Rd, Indianapolis, IN 46260; e-mail: ashapiro@ihtc.org.