Abstract
Integrin α2β1–mediated adhesion of human platelets to monomeric type I collagen or to the GFOGER peptide caused a time-dependent activation of PI3K and Akt phosphorylation. This process was abrogated by pharmacologic inhibition of PI3Kβ, but not of PI3Kγ or PI3Kα. Moreover, Akt phosphorylation was undetectable in murine platelets expressing a kinase-dead mutant of PI3Kβ (PI3KβKD), but occurred normally in PI3KγKD platelets. Integrin α2β1 failed to stimulate PI3Kβ in platelets from phospholipase Cγ2 (PLCγ2)–knockout mice, and we found that intracellular Ca2+ linked PLCγ2 to PI3Kβ activation. Integrin α2β1 also caused a time-dependent stimulation of the focal kinase Pyk2 downstream of PLCγ2 and intracellular Ca2+. Whereas activation of Pyk2 occurred normally in PI3KβKD platelets, stimulation of PI3Kβ was strongly reduced in Pyk2-knockout mice. Neither Pyk2 nor PI3Kβ was required for α2β1–mediated adhesion and spreading. However, activation of Rap1b and inside-out stimulation of integrin αIIbβ3 were reduced after inhibition of PI3Kβ and were significantly impaired in Pyk2-deficient platelets. Finally, both PI3Kβ and Pyk2 significantly contributed to thrombus formation under flow. These results demonstrate that Pyk2 regulates PI3Kβ downstream of integrin α2β1, and document a novel role for Pyk2 and PI3Kβ in integrin α2β1 promoted inside-out activation of integrin αIIbβ3 and thrombus formation.
Introduction
Class I PI3Ks are key signaling enzymes that phosphorylate the inositol ring of membrane phospholipids and generate different 3-phosphoinositides, important intracellular messengers that regulate several cellular processes through the downstream activation of the protein Ser/Thr kinase Akt.1 Circulating blood platelets express all members of the class I PI3K family, which includes the PI3Kα, PI3Kβ, PI3Kδ, and PI3Kγ isoforms. PI3K activity is essential for platelet aggregation and thrombus formation,1,2 and therefore these enzymes are potential novel targets for antithrombotic agents. For this reason, it is essential to recognize the precise contribution of every PI3K isoform in platelet activation induced by different extracellular agonists.
Pharmacologic and genetic evidence indicates that PI3Kβ plays a predominant role in the regulation of platelet function.3-7 Selective inactivation of PI3Kβ completely prevents platelet aggregation induced by the collagen receptor glycoprotein VI (GPVI) and reduces occlusive thrombus formation.4,5 PI3Kβ is also implicated in the platelet response to agonists that stimulate G-protein coupled receptors (GPCRs) such as ADP or thromboxane A2 (TxA2).3,4,8,9 Whereas PI3Kδ has been demonstrated to play a minor role in platelet activation,10 PI3Kα was recently proposed to be as important as PI3Kβ in GPVI signaling.7 Similarly, several reports have documented that, in addition to PI3Kβ, PI3Kγ is also implicated in GPCR-mediated platelet activation.4,9,11,12 These observations are indicative of a still poorly appreciated interplay between the different PI3K isoforms in selected contexts of platelet activation. PI3Kβ has also been proposed to be involved in integrin αIIbβ3–mediated outside-in signaling, a process essential for platelet spreading, stable thrombus formation, and clot retraction.3-5,13 In contrast, very little is known about the role and regulation of PI3K downstream of the other major platelet integrin, integrin α2β1.
Together with GPVI, integrin α2β1 is a platelet receptor for collagen,14 but it can also interact with other ligands, including decorin and tenascin.15,16 Although some controversies persist, the role of integrin α2β1 in adhesion to collagen, platelet activation, and thrombus formation is well documented.14,17-19 Recruitment of integrin α2β1 initiates an outside-in signaling pathway leading to platelet spreading on the extracellular matrix and to activation of integrin αIIbβ3, thus allowing binding of soluble fibrinogen to adherent platelets.20-23 The organization of this intracellular signaling pathway is still poorly understood. It is very well documented that phospholipase Cγ2 (PLCγ2) is activated, and leads to intracellular Ca2+ increase and protein kinase C (PKC) stimulation.20,21 PLCγ2 activation is essential for integrin α2β1–mediated spreading, as well as for the cross-talk with integrin αIIbβ3.20,21 It has also been shown that integrin α2β1 stimulates tyrosine kinases, including Src and Syk, as well as small GTPases such as Rac and Rap1b.20-24 Based on the observation that the PI3K inhibitor wortmannin affects some platelet responses,22,23 the stimulation of PI3K by integrin α2β1 has been hypothesized, but so far this has not been demonstrated directly.
In the present study, we adopted pharmacologic and genetic approaches to investigate the regulation and function of PI3K in integrin α2β1–mediated adhesion of human and murine platelets. We demonstrate that integrin α2β1 selectively stimulates the PI3Kβ isoform through a novel mechanism that involves intracellular Ca2+ and the Ca2+-regulated tyrosine kinase Pyk2. We also provide evidence that PI3Kβ is dispensable for integrin α2β1–mediated platelet spreading on collagen, but is required for the inside-out activation of integrin αIIbβ3.
Methods
Materials
Monomeric type I collagen was provided by Prof. M. E. Tira (University of Pavia, Pavia, Italy). The GFOGER peptide was provided by Dr R. Farndale (University of Cambridge, Cambridge, United Kingdom). PLCγ2–knockout mice were kindly provided by Dr J. Ihle (St Jude Children's Research Hospital, Memphis, TN) through Dr S. P. Watson (University of Birmingham, Birmingham, United Kingdom). Generation and characterization of PI3KβKD, PI3KγKD, and Pyk2-knockout mice was described previously.25-27 The use of mice for our experimental work was approved by the Ethics Committee of the University of Pavia. The rabbit polyclonal Abs against Rap1 (121), against Pyk2 (N-19), as well as the mAb anti-tubulin (DM1A) were from Santa Cruz Biotechnology. Anti–phospho-Akt(Ser473), and anti–phospho-Pyk2(Tyr402) Abs were from Cell Signaling Technology. Goat polyclonal anti-pleckstrin Ab was from Abcam. Apyrase, acetylsalicylic acid (ASA), TRITC-conjugated phalloidin, fibrinogen, AS252424, and CFSE were from Sigma-Aldrich. Biotinylated-fibrinogen was prepared as described previously.28 The bicinchoninic acid assay and the enhanced chemiluminescence substrate were from Pierce. RO318220 and 2-APB were from Calbiochem. Wortmannin and BAPTA-AM were from ALEXIS Biochemicals. TGX-221 was a gift from Dr Peter R. Shepherd (University of Auckland, Auckland, New Zealand). PIK-75 was from Axon MedChem.
Preparation of human and murine platelets
Human platelets were obtained from healthy volunteers who had not taken drugs for at least 2 weeks before the withdrawal; citric acid/citrate/dextrose as anticoagulant (152mM sodium citrate, 130mM citric acid, and 112mM glucose). Whole blood was centrifuged at 120g for 10 minutes at room temperature, and then apyrase (0.2 U/mL) and PGE1 (1μM) were added to the platelet-rich plasma. Platelets were recovered by centrifugation at 720g for 15 minutes, washed with 5 mL of PIPES buffer (20mM PIPES and 136mM NaCl, pH 6.5), and finally gently resuspended in HEPES buffer (10mM HEPES, 137mM NaCl, 2.9mM KCl, and 12mM NaHCO3, pH 7.4). The cell count was typically adjusted to 0.4 × 109 platelets/mL.
Murine platelets were prepared from blood collected from abdominal vena cava using citric acid/citrate/dextrose/3.8% Na-citrate (2:1) as an anticoagulant. Blood was centrifuged at 180g for 10 minutes, and then 0.02 U/mL of apyrase, 10μM indomethacin, and 1μM PGE1 were added to the isolated platelet-rich plasma. To increase platelet yield, the RBC pellet was washed with HEPES buffer and centrifuged at 180g for 10 minutes. The upper phase was collected and pooled with the platelet-rich plasma. Platelets were then recovered by centrifugation at 550g for 7 minutes, washed, and resuspended in HEPES buffer. The platelet count was adjusted to 0.3 × 109 cells/mL and after the addition of 5.5mM glucose, cells were allowed to rest for 30 minutes at room temperature.
Adhesion assay
Polystyrene dishes (60-mm) were coated overnight at room temperature with 50 μg/mL of monomeric type I collagen diluted in 0.1M acetic acid or 10 μg/mL of GFOGER peptide diluted in PBS. Dishes were washed 3 times with 5 mL of PBS, blocked with 2 mL of 1% BSA in PBS for 2 hours at room temperature, and then washed again 3 times with PBS. Human or murine platelets (0.5 mL at 4 × 108 platelets/mL for human platelets or 3 × 108 platelets/mL for murine platelets) were added to collagen-coated dishes in the presence of 2mM MgCl2 and 1 mg/mL of BSA. Apyrase (0.4 U/mL) was also typically added to the platelet suspension, as indicated in the “Results.” After 10, 30, or 60 minutes of incubation at room temperature, nonadherent cells were removed and dishes were washed 3 times with 5 mL of PBS. For whole-cell lysate preparation, adherent cells were solubilized directly by the addition of 0.5 mL of 2% SDS in HEPES buffer and then collected. For analysis of Rap1 activation, adherent platelets were recovered by lysis with 1 mL of ice-cold RIPA buffer (50mM Tris/HCl, pH 7.4, 200mM NaCl, 2.5mM MgCl2, 1% Nonidet P-40, 10% glycerol, 1mM PMSF, 1μM leupeptin, 0.1μM aprotinin, and 0.1μM Na3VO4) and lysates were centrifuged at 18 000g for 10 minutes. The protein content in the cleared supernatant was determined by the bicinchoninic acid assay, and aliquots of each sample containing the same amount of proteins (and, thus, deriving from the same number of adherent platelets) were used for immunoblotting analysis or for Rap1b activation assay.
Evaluation of platelet adhesion and spreading was performed using a fluorescence microscopy–based method after incubation of platelets on glass coverslips coated with 50 μg/mL of monomeric type I collagen in 0.1 M acetic acid. Adherent platelets were fixed, permeabilized, and stained by TRITC-conjugated phalloidin. Platelets were viewed on a fluorescence microscope (Olympus BX51), and digital images (40×) were acquired. The number of adherent cells, as well as the average cell area (as an index of platelet spreading), was determined using the ImageJ Version 1.42 software. For each specimen, 5 different fields were analyzed by 2 independent observers.
Rap1 activation assay
Analysis of accumulation of active GTP-bound Rap1b in adherent platelets was performed by a pull-down assay using the glutathione S-transferase–tagged Rap-binding domain of RalGDS, essentially as described previously.28
Electrophoresis and immunoblotting
Aliquots of platelet lysates containing the same amount of proteins were dissociated by addition of 0.5 volumes of SDS sample buffer 3× (37.5mM Tris, 288mM glycine, pH 8.3, 6% SDS, 1.5% DTT, 30% glycerol, and 0.03% bromophenol blue), and samples were heated at 95°C for 3 minutes. SDS-sample buffer 2× was also added to precipitated, active Rap1b. Proteins were separated by SDS-PAGE, typically on a 10% acrylamide gel for the analysis of Akt or Pyk2 phosphorylation, or on a 10%-20% acrylamide gradient gel for Rap1b detection, and subsequently transferred to PVDF membrane. Membranes were blocked for 2 hours with 5% BSA in TBS (20mM Tris/HCl, pH 7.5, 0.5mM NaCl), and then incubated overnight at 4°C with the desired primary Abs diluted in 20mM Tris/HCl, pH 7.5, and 0.5mM NaCl. In the present study, the following Abs and dilutions were used: anti–phospho-Akt(Ser473), 1:500; anti-phosphoPyk2(Tyr402), 1:500; anti-pleckstrin, 1:1000; anti-tubulin, 1:1000; anti-Pyk2, 1:500; anti-FAK, 1:200; and anti-Rap1, 1:1000. Membranes were then extensively washed with 0.1% Tween 20 in TBS, and incubated with peroxidase-conjugated secondary Ab (1:3000 dilution) for 45 minutes. After extensive washing, reactive proteins were visualized with a chemiluminescence reaction. The PVDF membranes were then stripped and reprobed with a different Ab (typically, anti-tubulin or anti-pleckstrin) as a control for equal loading. All of the immunoblots are representative of at least 3 different experiments giving similar results. Quantification of protein intensity was performed by computer-assisted densitometric scanning using ImageJ Version 1.42 software.
Thrombus formation under flow
Glass coverslips were coated with monomeric type I collagen (100 μg/mL) and blocked with 1% BSA in PBS, pH 7.4. The coverslips were mounted in a 50-μm-deep parallel-plate flow chamber (RC-31 from Warner Instruments) under a fluorescence microscope, and rinsed with washing buffer (HEPES buffer supplemented with 2mM CaCl2, 2mM MgCl2, 5.5mM glucose, 0,1% BSA, and 1 U/mL of heparin). PPACK/heparin–treated mouse blood was preincubated with 3 μg/mL of CFSE for 5 minutes and flowed over collagen at 1000/s for 4 minutes using a pump system (Harvard Apparatus PHD 2000). After perfusion, the flow chamber was rinsed with washing buffer, and at least 10 randomly taken fluorescence microscopic images were collected after 2 minutes and 10 minutes of rinse. Images were analyzed by ImageJ Version 1.92 software and the extent of thrombus formation was calculated as the percentage of platelet covered area.
Measurement of fibrinogen binding to collagen adherent platelets
Measurement and quantification of specific binding of biotin-labeled fibrinogen to integrin αIIbβ3 in platelets adherent through integrin α2β1 was performed according to a procedure that integrates data from multiple determination, as described previously.21 This procedure allows the calculation of the specific binding of fibrinogen for the same number of adherent cells.
Results
Integrin α2β1 activates PI3Kβ
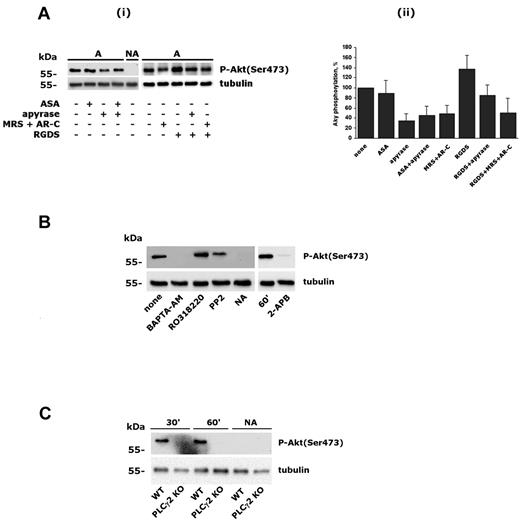
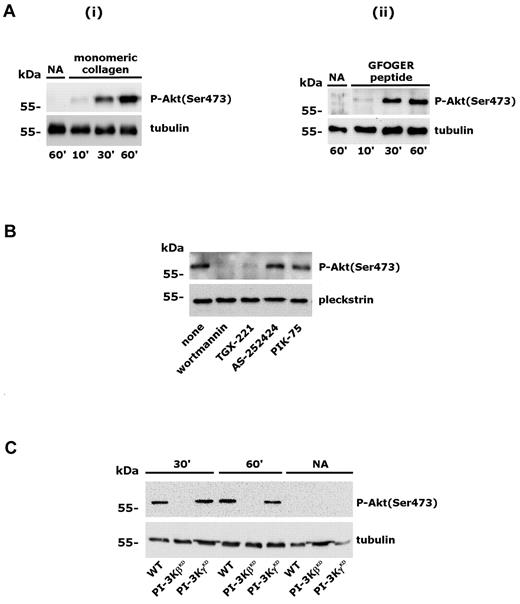
We reported previously that PI3Kβ is essential for GPVI-mediated platelet activation and is required for platelet spreading on fibrinogen.4 In the present study, we investigated the activation of PI3K on platelet adhesion through integrin α2β1 by measuring the phosphorylation of the downstream effector Akt. Washed human platelets were allowed to adhere to immobilized monomeric type I collagen or to GFOGER peptide in the presence of 2mM MgCl2 for increasing times. We demonstrated previously that, under the conditions of this assay, monomeric type I collagen promotes platelet adhesion exclusively through integrin α2β1 and does not lead to GPVI stimulation.4,24,29 GFOGER peptide is a well-characterized specific ligand for integrin α2β1.30 Adherent platelets were lysed and Akt phosphorylation on Ser473 was evaluated by immunoblotting with a phosphospecific Ab. Figure 1A shows that engagement of integrin α2β1 by monomeric collagen or by GFOGER peptide induced a robust, time-dependent phosphorylation of Akt. To identify the PI3K isoform involved, platelets were incubated with inhibitors of different PI3K isoforms before adhesion to monomeric type I collagen. Figure 1B shows that integrin α2β1–induced Akt phosphorylation was prevented by wortmannin and by TGX-221, a selective inhibitor of PI3Kβ, but was unaffected by the PI3Kγ inhibitor AS252424 or by the PI3Kα inhibitor PIK-75. These results indicate that integrin α2β1 activates PI3Kβ. To confirm this observation, we analyzed Akt phosphorylation in murine platelets expressing catalytically inactive forms of either PI3Kβ (PI3KβKD) or PI3Kγ (PI3KγKD). Figure 1C shows that Akt phosphorylation induced by platelet adhesion through integrin α2β1 occurred normally in PI3KγKD platelets, but was not detectable in the absence of PI3Kβ activity. These results demonstrated that PI3Kβ is the PI3K isoform that is activated downstream of integrin α2β1 and is responsible for Akt phosphorylation in adherent platelets.
Activation of PI3Kβ by integrin α2β1 engagement. (A) Human platelets were allowed to adhere to immobilized monomeric type I collagen (i) or GFOGER peptide (ii) for 10, 30, or 60 minutes. Adherent cells and nonadherent cells (NA) from the 60-minute samples were collected and lysed. Akt phosphorylation was analyzed by immunoblotting with anti–phospho-Akt(Ser473) Ab (top rows). Equal loading of the samples was verified by subsequent immunoblotting with anti-tubulin (bottom rows). (B) Human platelets were preincubated with DMSO, wortmannin (100nM, 15 minutes), TGX-221 (0.5μM, 10 minutes), AS252424 (0.5μM, 10 minutes), or PIK-75 (0.5μM, 10 minutes), and Akt phosphorylation (top row) was evaluated by immunoblotting in platelets adherent to monomeric type I collagen for 60 minutes. Subsequent analysis of pleckstrin levels (bottom row) was performed to control loading of the samples. (C) Wild-type murine platelets (WT) and platelets from PI3KγKD or PI3KβKD mice were allowed to adhere to monomeric collagen through integrin α2β1 for 30 or 60 minutes, as indicated. Nonadherent platelets (NA) were also collected after 60 minutes. The top row shows Akt phosphorylation in adherent and nonadherent platelets, and the bottom row shows the comparable expression of tubulin in all samples.
Activation of PI3Kβ by integrin α2β1 engagement. (A) Human platelets were allowed to adhere to immobilized monomeric type I collagen (i) or GFOGER peptide (ii) for 10, 30, or 60 minutes. Adherent cells and nonadherent cells (NA) from the 60-minute samples were collected and lysed. Akt phosphorylation was analyzed by immunoblotting with anti–phospho-Akt(Ser473) Ab (top rows). Equal loading of the samples was verified by subsequent immunoblotting with anti-tubulin (bottom rows). (B) Human platelets were preincubated with DMSO, wortmannin (100nM, 15 minutes), TGX-221 (0.5μM, 10 minutes), AS252424 (0.5μM, 10 minutes), or PIK-75 (0.5μM, 10 minutes), and Akt phosphorylation (top row) was evaluated by immunoblotting in platelets adherent to monomeric type I collagen for 60 minutes. Subsequent analysis of pleckstrin levels (bottom row) was performed to control loading of the samples. (C) Wild-type murine platelets (WT) and platelets from PI3KγKD or PI3KβKD mice were allowed to adhere to monomeric collagen through integrin α2β1 for 30 or 60 minutes, as indicated. Nonadherent platelets (NA) were also collected after 60 minutes. The top row shows Akt phosphorylation in adherent and nonadherent platelets, and the bottom row shows the comparable expression of tubulin in all samples.
PI3Kβ is regulated by intracellular Ca2+ downstream of integrin α2β1
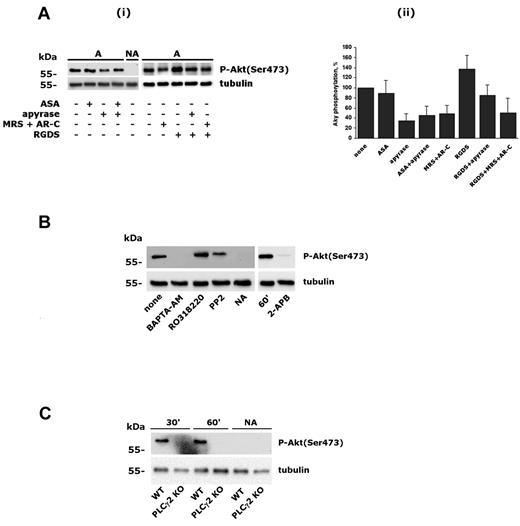
It is known that PI3Kβ can be activated by GPCRs.1,3,4 Therefore, we considered that released ADP and/or generated TxA2 could contribute to Akt phosphorylation on platelet adhesion through integrin α2β1. Figure 2A shows that Akt phosphorylation was unaltered in ASA-treated platelets, indicating that TxA2 does not contribute to PI3K activation. In contrast, neutralization of secreted ADP by apyrase, as well as blockade of both P2Y1 and P2Y12 receptor with the selective antagonists MRS2179 and AR-C69931MX, strongly reduced, but did not abolish, integrin α2β1–induced phosphorylation of Akt. When added together, apyrase and ASA had no additive effects. Therefore, secreted ADP, but not TxA2, contributed to integrin-mediated PI3Kβ activation. Phosphorylation of Akt induced by integrin α2β1–mediated platelet adhesion was not reduced in the presence of RGDS, rather, a small increase was detected and was still partially sensitive to apyrase and ADP receptor antagonists (Figure 2A). These results exclude a significant contribution of platelet autocrine stimulation by integrin αIIbβ3 due to binding of potentially secreted fibrinogen. To focus on the direct link between integrin α2β1 and PI3Kβ, all subsequent experiments were performed in the presence of apyrase.
Characterization of integrin α2β1–induced PI3Kβ activation. (A) Role of secreted ADP, TxA2, and integrin αIIbβ3 in integrin α2β1–triggered Akt phosphorylation. Platelets were incubated with 0.5mM ASA for 15 minutes, with 2 U/mL of apyrase, 0.5mM RGDS, or a mixture of 100μM MRS2179 and 0.5μM AR-C69931MX for 2 minutes, and then allowed to adhere to monomeric collagen for 60 minutes. Nonadherent platelets from untreated samples (NA) were also collected. In panel i, the top row shows a typical immunoblot with anti–phospho-Akt(Ser 473) Ab, and the bottom row shows the level of tubulin in the different samples. Panel ii shows a quantitative evaluation of Akt phosphorylation performed by densitometric analysis of immunoblots. Data are the means ± SD of 3 different experiments. (B) Analysis of Akt phosphorylation in platelets after 60 minutes of adhesion after treatment with BAPTA-AM (20μM, 30 minutes), RO318220 (10μM, 5 minutes), PP2 (20μM, 15 minutes), or 2-APB (100μM, 10 minutes), as indicated. As a negative control, nonadherent platelets (NA) from untreated samples (none) were also analyzed. Subsequent immunoblotting with anti-tubulin (bottom row) was performed as control for equal loading. (C) Analysis of Akt phosphorylation in murine platelets from wild-type (WT) and PLCγ2–knockout (PLCγ2 KO) mice. Adherent platelets were recovered after 30 and 60 minutes, as indicated on the top. Nonadherent cells were analyzed after 60 minutes.
Characterization of integrin α2β1–induced PI3Kβ activation. (A) Role of secreted ADP, TxA2, and integrin αIIbβ3 in integrin α2β1–triggered Akt phosphorylation. Platelets were incubated with 0.5mM ASA for 15 minutes, with 2 U/mL of apyrase, 0.5mM RGDS, or a mixture of 100μM MRS2179 and 0.5μM AR-C69931MX for 2 minutes, and then allowed to adhere to monomeric collagen for 60 minutes. Nonadherent platelets from untreated samples (NA) were also collected. In panel i, the top row shows a typical immunoblot with anti–phospho-Akt(Ser 473) Ab, and the bottom row shows the level of tubulin in the different samples. Panel ii shows a quantitative evaluation of Akt phosphorylation performed by densitometric analysis of immunoblots. Data are the means ± SD of 3 different experiments. (B) Analysis of Akt phosphorylation in platelets after 60 minutes of adhesion after treatment with BAPTA-AM (20μM, 30 minutes), RO318220 (10μM, 5 minutes), PP2 (20μM, 15 minutes), or 2-APB (100μM, 10 minutes), as indicated. As a negative control, nonadherent platelets (NA) from untreated samples (none) were also analyzed. Subsequent immunoblotting with anti-tubulin (bottom row) was performed as control for equal loading. (C) Analysis of Akt phosphorylation in murine platelets from wild-type (WT) and PLCγ2–knockout (PLCγ2 KO) mice. Adherent platelets were recovered after 30 and 60 minutes, as indicated on the top. Nonadherent cells were analyzed after 60 minutes.
It is known that outside-in signaling through integrin α2β1 involves PLCγ2 activation, leading to stimulation of PKC and intracellular Ca2+ increase.20,21 We showed recently that integrin α2β1–mediated PLCγ2 activation occurs through Src-dependent and Src-independent mechanisms.29 Therefore, we investigated the contribution of Src kinase and intracellular messengers generated by PLCγ2 on PI3Kβ activation. Figure 2B shows that inhibition of Src kinases by PP2 only partially prevented PI3Kβ activation (22 ± 3 of inhibition, n = 3). In contrast, Akt phosphorylation was completely suppressed by intracellular Ca2+ chelation with BAPTA-AM, but not after inhibition of PKC with RO318220. Interestingly, Akt phosphorylation was also suppressed by 2-APB, an inhibitor of the IP3 receptor and thus an antagonist of IP3-mediated Ca2+ release. These findings place PI3Kβ downstream of PLC and intracellular Ca2+ in integrin α2β1 signaling. Therefore, we analyzed PI3Kβ activation in platelets from PLCγ2–knockout mice. Figure 3C shows that engagement of integrin α2β1 in PLCγ2–deficient platelets failed to induce Akt phosphorylation, indicating that PLCγ2 is required for PI3Kβ stimulation.
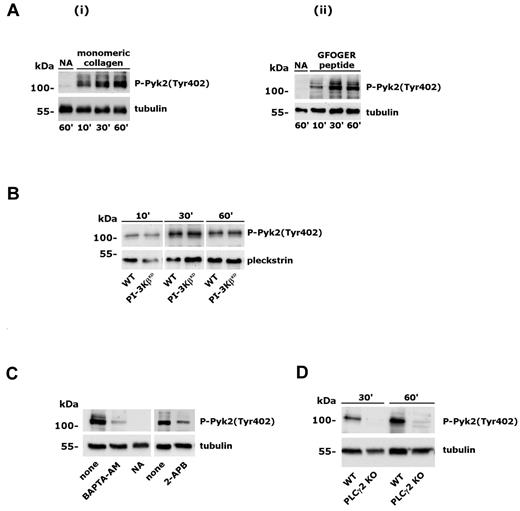
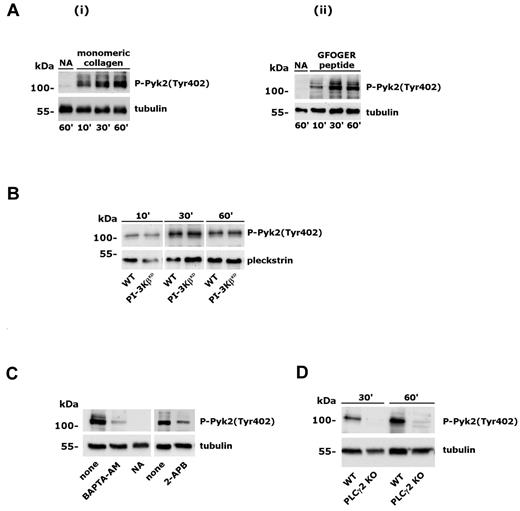
Analysis of Pyk2 phosphorylation induced by integrin α2β1. Adhesion-induced Pyk2 phosphorylation was evaluated on whole-platelet lysates by immunoblotting with anti–phospho-Pyk2(Tyr402) Ab (top rows). Subsequent staining with anti-tubulin or anti-pleckstrin Ab is reported in the bottom rows as a control for equal loading. (A) Platelet adhesion to monomeric collagen (i) or GFOGER peptide (ii) was performed for the times indicated on the bottom. Samples of nonadherent platelets were collected after 60 minutes. (B) Analysis of Pyk2 phosphorylation in wild-type and PI3KβKD murine platelets adherent to monomeric collagen for the 10, 30, or 60 minutes. (C) Effect of platelet incubation with BAPTA-AM (20μM, 30 minutes) or 2-APB (100μM, 10 minutes) on integrin α2β1–induced Pyk2 phosphorylation. Platelet adhesion was performed for 60 minutes. None indicates control platelets treated with DMSO; NA, nonadherent, untreated platelets. (D) Analysis of Pyk2 phosphorylation in platelets from wild-type (WT) and PLCγ2–knockout (PLCγ2 KO) mice after adhesion to monomeric collagen for 30 and 60 minutes.
Analysis of Pyk2 phosphorylation induced by integrin α2β1. Adhesion-induced Pyk2 phosphorylation was evaluated on whole-platelet lysates by immunoblotting with anti–phospho-Pyk2(Tyr402) Ab (top rows). Subsequent staining with anti-tubulin or anti-pleckstrin Ab is reported in the bottom rows as a control for equal loading. (A) Platelet adhesion to monomeric collagen (i) or GFOGER peptide (ii) was performed for the times indicated on the bottom. Samples of nonadherent platelets were collected after 60 minutes. (B) Analysis of Pyk2 phosphorylation in wild-type and PI3KβKD murine platelets adherent to monomeric collagen for the 10, 30, or 60 minutes. (C) Effect of platelet incubation with BAPTA-AM (20μM, 30 minutes) or 2-APB (100μM, 10 minutes) on integrin α2β1–induced Pyk2 phosphorylation. Platelet adhesion was performed for 60 minutes. None indicates control platelets treated with DMSO; NA, nonadherent, untreated platelets. (D) Analysis of Pyk2 phosphorylation in platelets from wild-type (WT) and PLCγ2–knockout (PLCγ2 KO) mice after adhesion to monomeric collagen for 30 and 60 minutes.
Role of the tyrosine kinase Pyk2 on Ca2+-dependent stimulation of PI3Kβ
Multiple mechanisms have been proposed to activate PI3Kβ, including binding to phosphorylated tyrosine kinases through the SH2 domain–containing regulatory subunit, G-protein βγ dimers, and Ras.1 To characterize the mechanism for the novel Ca2+-mediated regulation of PI3Kβ in platelet integrin α2β1 signaling, we hypothesized the involvement of the focal adhesion kinase Pyk2, which is known to be activated both by Src-dependent phosphorylation and by intracellular Ca2+.31 Pyk2 is expressed in platelets and is activated by several soluble agonists.32-34 However, its implication in integrin α2β1 signaling is still unknown. Using a phospho-specific Ab able to detect Pyk2 autophosphorylation on Tyr402, we evaluated the activation of this kinase in platelets adherent to monomeric collagen or to GFOGER peptide. Figure 3A shows that integrin α2β1 promoted the time-dependent activation and autophosphorylation of Pyk2. Activation of Pyk2 did not require PI3K activity, because it occurred normally in the PI3KβKD platelets (Figure 3B), but was regulated by intracellular Ca2+, because it was inhibited by BAPTA-AM and after blockade of IP3-mediated Ca2+ release by 2-APB (Figure 3C). Integrin α2β1–induced activation of Pyk2 was completely dependent on PLCγ2, because it failed to occur in PLCγ2–deficient platelets (Figure 3D). Therefore, as for PI3Kβ, Pyk2 activation is also downstream of PLCγ2 and cytosolic Ca2+.
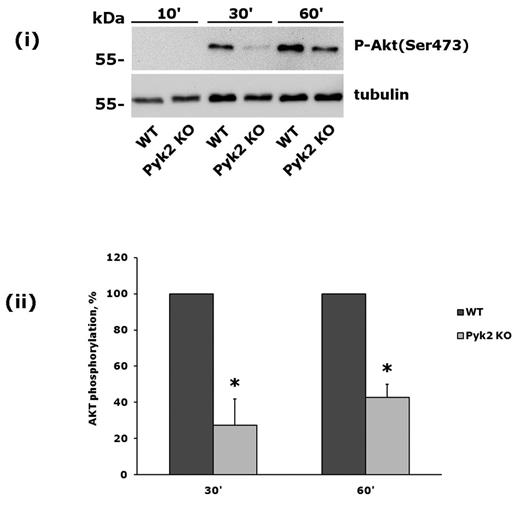
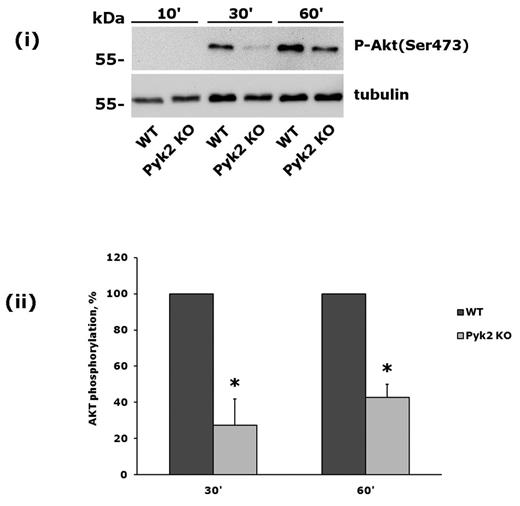
To further investigate the role of Pyk2 in PI3Kβ activation, we analyzed platelets from Pyk2-knockout mice. These cells did not express Pyk2, but contained normal amounts of the related focal adhesion kinase FAK (data not shown). Pyk2-knockout platelets were allowed to adhere to monomeric collagen, and phosphorylation of Akt was evaluated after 30 and 60 minutes. We observed a strong and statistically significant inhibition of integrin α2β1–mediated phosphorylation of Akt in Pyk2-deficient platelets (Figure 4). Therefore we conclude that the Ca2+-dependent tyrosine kinase Pyk2 links PLCγ2 activation to PI3Kβ downstream of integrin α2β1.
Integrin α2β1–induced PI3Kβ activation is impaired in Pyk2-deficient platelets. Comparative analysis of Akt phosphorylation in wild-type and Pyk2-knockout platelets on adhesion to monomeric collagen for 10, 30, and 60 minutes. A representative immunoblot is shown in panel A. Quantification of Akt phosphorylation, performed by densitometric analysis of the immunoreactive bands, is shown in panel B. Black bars are wild-type platelets, gray bars are Pyk2-knockout platelets. Data are the means ± SD of 3 different experiments. *P < .05.
Integrin α2β1–induced PI3Kβ activation is impaired in Pyk2-deficient platelets. Comparative analysis of Akt phosphorylation in wild-type and Pyk2-knockout platelets on adhesion to monomeric collagen for 10, 30, and 60 minutes. A representative immunoblot is shown in panel A. Quantification of Akt phosphorylation, performed by densitometric analysis of the immunoreactive bands, is shown in panel B. Black bars are wild-type platelets, gray bars are Pyk2-knockout platelets. Data are the means ± SD of 3 different experiments. *P < .05.
PI3Kβ is required for the cross-talk between integrin α2β1 and integrin αIIbβ3
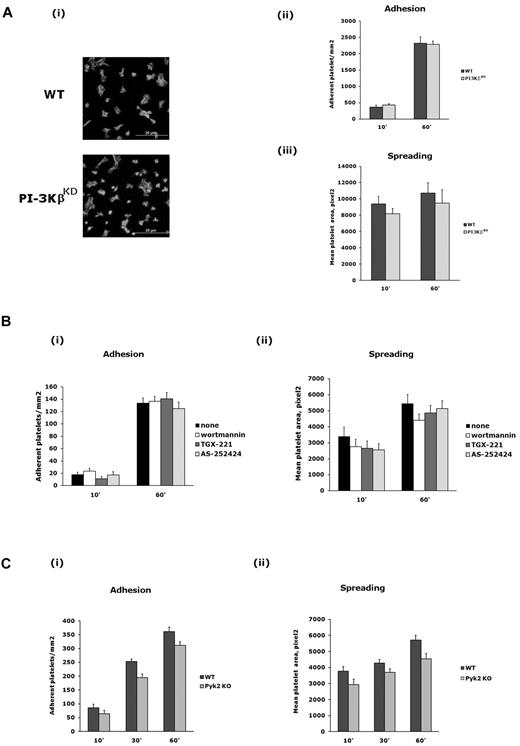
We next investigated the functional relevance of the Pyk2/PI3Kβ pathway in platelet adhesion through integrin α2β1. We showed previously that PI3Kβ is required for platelet spreading on fibrinogen.4 In contrast, we failed to detect any significant difference between wild-type and PI3KβKD platelets in platelet adhesion or spreading mediated by integrin α2β1 (Figure 5A). This observation was also supported by pharmacologic studies with specific inhibitors. As shown in Figure 5B, integrin α2β1–mediated platelet adhesion and spreading were not affected by wortmannin, TGX-221, or AS252424. Similarly, no significant differences in integrin α2β1–mediated adhesion and spreading were detected between wild-type and Pyk2-deficient platelets (Figure 5C), indicating that, as was the case for PI3Kβ, Pyk2 is also not required for these processes.
Role of PI3Kβ and Pyk2 in platelet adhesion and spreading through integrin α2β1. (A) Wild-type and PI3KβKD platelets were allowed to adhere to immobilized monomeric collagen for 10 or 60 minutes. Adherent cells were permeabilized, stained with TRITC-phalloidin, and adhesion (as number of cells/mm2) and spreading (as mean platelet area) were evaluated as described in “Methods.” (i) Representative image of adherent wild-type (WT) and PI3KβKD platelets after 60 minutes (40× amplification). Quantification of adhesion and spreading is reported in panels ii and iii, respectively. Data are the means ± SD of 3 different experiments. (B) Integrin α2β1–mediated adhesion (i) and spreading (ii) of platelets preincubated with wortmannin (100nM, 15 minutes), TGX-221 (0.5μM, 10 minutes), or AS252424 (0.5mM, 10 minutes), as indicated on the right, after 10 or 60 minutes, as indicated on the bottom. Results are expressed as means ± SD of 3 different experiments. (C) Integrin α2β1–mediated adhesion (i) and spreading (ii) of platelets from wild-type (WT) and Pyk2-knockout (Pyk2 KO) after 10, 30, or 60 minutes. Results are expressed as means ± SD of 3 different experiments.
Role of PI3Kβ and Pyk2 in platelet adhesion and spreading through integrin α2β1. (A) Wild-type and PI3KβKD platelets were allowed to adhere to immobilized monomeric collagen for 10 or 60 minutes. Adherent cells were permeabilized, stained with TRITC-phalloidin, and adhesion (as number of cells/mm2) and spreading (as mean platelet area) were evaluated as described in “Methods.” (i) Representative image of adherent wild-type (WT) and PI3KβKD platelets after 60 minutes (40× amplification). Quantification of adhesion and spreading is reported in panels ii and iii, respectively. Data are the means ± SD of 3 different experiments. (B) Integrin α2β1–mediated adhesion (i) and spreading (ii) of platelets preincubated with wortmannin (100nM, 15 minutes), TGX-221 (0.5μM, 10 minutes), or AS252424 (0.5mM, 10 minutes), as indicated on the right, after 10 or 60 minutes, as indicated on the bottom. Results are expressed as means ± SD of 3 different experiments. (C) Integrin α2β1–mediated adhesion (i) and spreading (ii) of platelets from wild-type (WT) and Pyk2-knockout (Pyk2 KO) after 10, 30, or 60 minutes. Results are expressed as means ± SD of 3 different experiments.
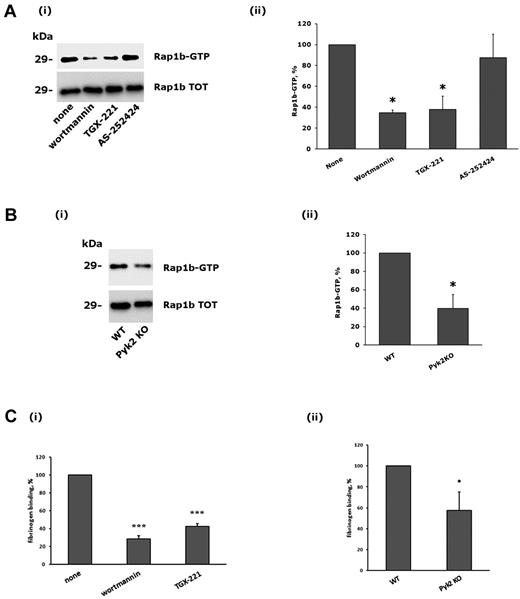
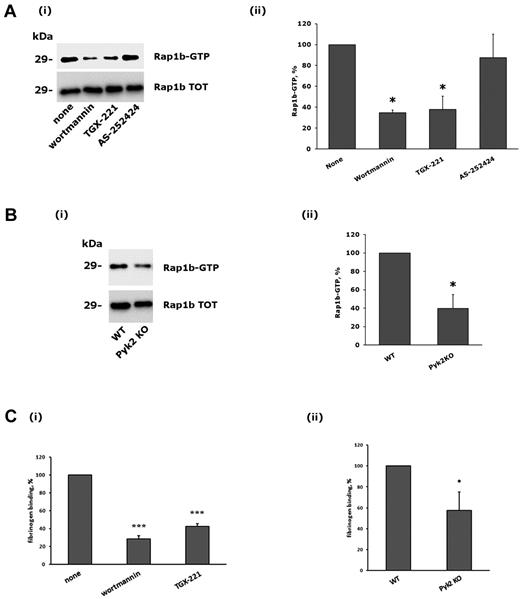
Platelet adhesion through integrin α2β1 leads to the inside-out activation of integrin αIIbβ3 through a signaling pathway that involves PLCγ2 and the small GTPase Rap1b.21,29 Therefore, we investigated the contribution of Pyk2 and PI3Kβ on the cross-talk to integrin αIIbβ3. Figure 6A shows that adhesion-dependent activation of Rap1b was strongly reduced after inhibition of PI3Kβ by wortmannin or TGX-221. Moreover, a similarly impaired Rap1b activation induced by integrin α2β1 was observed in the Pyk2-deficient platelets. Therefore, Pyk2 and PI3Kβ are important regulators of Rap1b activity downstream of integrin α2β1.
Role of PI3Kβ and Pyk2 in integrin α2β1–mediated Rap1b stimulation and integrin αIIbβ3 activation. (A) Analysis of Rap1b activation. Active GTP-bound Rap1b was precipitated from platelets that had been allowed to adhere to monomeric collagen for 60 minutes after incubation with DMSO (none), wortmannin (100nM, 15 minutes), TGX-221 (0.5μM, 10 minutes), or AS252424 (0.5μM, 10 minutes), as indicated on the bottom. A representative immunoblot is reported in panel i, where the top row shows the active form of Rap1b and the bottom row the level of total Rap1b present in the platelet lysates. Quantification of Rap1b activity was performed by densitometric analysis of the immunoblots, and the results are reported in panel ii. The amount of active Rap1b in adherent DMSO-treated platelets was taken as 100%. Data are the means ± SD of 3 different experiments. *P < .05. (B) Comparative analysis of Rap1b activation in wild-type (WT) and Pyk2-deficient platelets (Pyk2 KO) after adhesion to monomeric collagen for 60 minutes. Both a representative immunoblot (i), and quantitative analysis (ii) of Rap1b activation are shown. Data in panel ii are the means ± SD of 3 different experiments. *P < .05. (C) Analysis of specific binding of biotinylated fibrinogen to adherent platelets. The effect of preincubation of platelets with wortmannin (100nM, 15 minutes), or TGX-221 (0.5μM, 10 minutes) is reported in panel i, where the binding of fibrinogen to DMSO-treated control platelets was taken as 100%. The comparative binding of fibrinogen to adherent platelets from wild-type (WT) or Pyk2-knockout (Pyk2 KO) mice is reported in panel ii. In both cases, data are the means ± SD of 4 different experiments. *P < .05; ***P < .001.
Role of PI3Kβ and Pyk2 in integrin α2β1–mediated Rap1b stimulation and integrin αIIbβ3 activation. (A) Analysis of Rap1b activation. Active GTP-bound Rap1b was precipitated from platelets that had been allowed to adhere to monomeric collagen for 60 minutes after incubation with DMSO (none), wortmannin (100nM, 15 minutes), TGX-221 (0.5μM, 10 minutes), or AS252424 (0.5μM, 10 minutes), as indicated on the bottom. A representative immunoblot is reported in panel i, where the top row shows the active form of Rap1b and the bottom row the level of total Rap1b present in the platelet lysates. Quantification of Rap1b activity was performed by densitometric analysis of the immunoblots, and the results are reported in panel ii. The amount of active Rap1b in adherent DMSO-treated platelets was taken as 100%. Data are the means ± SD of 3 different experiments. *P < .05. (B) Comparative analysis of Rap1b activation in wild-type (WT) and Pyk2-deficient platelets (Pyk2 KO) after adhesion to monomeric collagen for 60 minutes. Both a representative immunoblot (i), and quantitative analysis (ii) of Rap1b activation are shown. Data in panel ii are the means ± SD of 3 different experiments. *P < .05. (C) Analysis of specific binding of biotinylated fibrinogen to adherent platelets. The effect of preincubation of platelets with wortmannin (100nM, 15 minutes), or TGX-221 (0.5μM, 10 minutes) is reported in panel i, where the binding of fibrinogen to DMSO-treated control platelets was taken as 100%. The comparative binding of fibrinogen to adherent platelets from wild-type (WT) or Pyk2-knockout (Pyk2 KO) mice is reported in panel ii. In both cases, data are the means ± SD of 4 different experiments. *P < .05; ***P < .001.
We next evaluated the inside-out activation of integrin αIIbβ3 by measuring the specific binding of fibrinogen to collagen-adherent platelets. Figure 6C shows that fibrinogen binding was strongly inhibited by wortmannin and by the specific PI3Kβ inhibitor TGX-221, and was also significantly reduced in the absence of Pyk2. Therefore, we conclude that Pyk2 and PI3Kβ play a role in the cross-talk between integrins α2β1and αIIbβ3.
Under flow conditions, activation of integrin αIIbβ3 in collagen-adherent platelets is important for the growth and stabilization of the thrombus. Therefore, we analyzed the role of Pyk2 and PI3Kβ in thrombus formation under flow. Fluorescently labeled platelets in whole blood were perfused for 4 minutes at a shear rate of 1000/s over immobilized monomeric collagen to favor integrin α2β1–initiated platelet adhesion and thrombus formation. The stability of the formed thrombus was then evaluated after secondary perfusion with HEPES buffer for 10 minutes. Figure 7 shows that thrombus formation was strongly reduced when blood from either Pyk2-deficient or PI3KβKD mice was perfused. The defective thrombus formation was more evident in the absence of catalytically active PI3Kβ (89.28% ± 0.67% reduction of the covered area compared with control, n = 4), than in the absence of Pyk2 (69.06% ± 7.27% reduction, n = 4). After extensive perfusion of buffer, we did not detect any significant reduction of the area covered by platelets in wild-type or Pyk2-knockout mice under our experimental conditions. However, a small but significant reduction of the covered area was detected in the absence of catalytically active PI3Kβ, which more likely reflects the detachment of adherent platelets, because basically no thrombi of relevant size were detected in these samples. These results indicate that perfusion of blood over monomeric collagen triggers the formation of stable platelet thrombi, which is supported by the Pyk2/PI3Kβ signaling pathway
Defective thrombus formation in the absence of Pyk2 or catalytically active PI3Kβ. CSFE-labeled platelets in whole blood from wild-type (WT), Pyk2 KO, and PI3KβKD mice were perfused over immobilized monomeric collagen at a shear rate of 1000/s for 4 minutes. Images were taken after brief rinse of the coverslips with washing buffer (2 minutes) and are reported in the top rows (A). Thrombus formation on the coverslips was evaluated by measuring the covered area in 10 different and randomly taken microscopic fields and results are reported in the histogram in the bottom row (B, black bars) as the means ± SD of 4 different experiments. Coverslips were then further perfused with washing buffer for 10 minutes, and additional images were taken to evaluate thrombus stability. The remaining area covered by thrombi on extensive washing is reported in the histogram in the bottom row (B, white bars) as the means ± SD of 4 different experiments.
Defective thrombus formation in the absence of Pyk2 or catalytically active PI3Kβ. CSFE-labeled platelets in whole blood from wild-type (WT), Pyk2 KO, and PI3KβKD mice were perfused over immobilized monomeric collagen at a shear rate of 1000/s for 4 minutes. Images were taken after brief rinse of the coverslips with washing buffer (2 minutes) and are reported in the top rows (A). Thrombus formation on the coverslips was evaluated by measuring the covered area in 10 different and randomly taken microscopic fields and results are reported in the histogram in the bottom row (B, black bars) as the means ± SD of 4 different experiments. Coverslips were then further perfused with washing buffer for 10 minutes, and additional images were taken to evaluate thrombus stability. The remaining area covered by thrombi on extensive washing is reported in the histogram in the bottom row (B, white bars) as the means ± SD of 4 different experiments.
Discussion
In the present study, we investigated the role and regulation of PI3K in platelet integrin α2β1 signaling. We have demonstrated that integrin α2β1 selectively stimulates PI3Kβ downstream of PLCγ2 through a mechanism that involves the Ca2+-dependent tyrosine kinase Pyk2. Moreover, we have shown that PI3Kβ is not required for integrin α2β1–mediated spreading, but is important for activation of the small GTPase Rap1b and for cross-talk to integrin αIIbβ3, leading to fibrinogen binding to collagen-adherent platelets.
Although the role of integrin α2β1 in platelet adhesion to collagen and its ability to trigger platelet activation are well documented, little is known about the outside-in signaling pathways activated by this integrin. For example, the involvement of PI3K has been hypothesized based on indirect evidence with inhibitors,22,23 but has never been documented directly. We filled this gap in information by measuring Akt phosphorylation, demonstrating the effective stimulation of PI3K by integrin α2β1, and also identified the isoform implicated.
Monomeric type I collagen has been mainly used in this study as a reliable, cheap, and easy-to-obtain ligand for integrin α2β1, because previous studies have clearly demonstrated that under these conditions no activation of GPVI occurs.4,24,29 However, stimulation of PI3Kβ activity by integrin α2β1 was also confirmed using a different specific ligand, the collagen-related peptide GFOGER. Moreover, although the majority of the experiments reported in this study were performed using type I monomeric collagen as an integrin α2β1 ligand, many of the results have been confirmed in experiments with the GFOGER peptide (data not shown).
Using a combination of pharmacologic and genetic approaches, we have identified PI3Kβ as the PI3K isoform stimulated by integrin α2β1. Among all of the members of the class I PI3Ks, PI3Kβ is emerging as a major regulator of platelet activation. We and others have shown previously that PI3Kβ is activated by GPVI and by GPCRs and is important for platelet spreading on fibrinogen.3-7 The finding that PI3Kβ is also activated by integrin α2β1 further extends the role and importance of this isoform in platelet function. PI3Kβ is also stimulated downstream of the P2Y12 receptor for ADP,3,4,8 and we have demonstrated herein that secreted ADP actually contributes to integrin α2β1–mediated Akt phosphorylation. However, we have also demonstrated that integrin α2β1 can stimulate PI3Kβ directly even in the absence of secondary released agonists, confirming that PI3Kβ can also be regulated directly by integrin α2β1 engagement.
A central event in platelet adhesion through integrin α2β1 is the stimulation of PLCγ2. Analysis of platelets from PLCγ2–knockout mice revealed that PLCγ2 is absolutely required for integrin α2β1–induced stimulation of PI3Kβ. Moreover, we found that PLCγ2 regulation of PI3Kβ occurs through intracellular Ca2+ increase rather than through PKC activation. Our findings are supported by a recent study reporting that Ca2+ is implicated in P2Y12-independent PI3K activation in thrombin-stimulated platelets.35 Our results expand these observations, because we have demonstrated herein that Ca2+-dependent activation of PI3K occurs in integrin signaling and we have identified PI3Kβ as the Ca2+-regulated PI3K isoform. Interestingly, when platelets are stimulated through the other main collagen receptor, GPVI, PI3Kβ stimulation lies upstream of PLCγ2, and is actually required for efficient PLCγ2 activation.36 In contrast, our results demonstrate that in integrin α2β1 outside-in signaling, engagement of PI3Kβ is completely downstream of PLCγ2. We also observed that, in this context, the PI3K inhibitor wortmannin did not affect PLCγ2 activation induced by integrin α2β1 (data not shown). These observations outline another important difference in the signaling pathways activated by the 2 main platelet collagen receptors. Although PLCγ2 is typically considered to be activated through Src-mediated phosphorylation, we have demonstrated previously that integrin α2β1 adopts multiple mechanisms and is able to stimulate PLCγ2 even in the absence of Src-mediated phosphorylation.29 Consistent with these findings, inhibition of Src kinase by PP2 reduced, but did not abolish, PI3Kβ activation. In the experimental model adopted in this study, PLCγ2–dependent activation of PI3Kβ can be detected only after 30 minutes of adhesion, whereas these processes are supposed to occur within seconds in vivo. This consideration, which also holds true for a large number of previous studies, clearly represents an intrinsic limitation in the investigation of the signaling processes associated with platelet adhesion. Further, the relevance of our experimental observations relies on the assumption that those events that in vitro need time to reach detectable levels actually occur much more rapidly under physiologic conditions in vivo.
PI3Kβ is typically considered to be activated by receptor or nonreceptor tyrosine kinases, which provide phosphotyrosine residues able to bind the SH2 domains of the regulatory subunit p85, thus relieving a constitutive inhibitory action on the p110β catalytic subunit.1 In addition, PI3Kβ has been found to be activated by G-proteins βγ dimers, and by the small GTPase Ras, through mechanisms that are not yet completely understood.1 In the present study, we propose a novel mechanism for PI3Kβ stimulation that involves elevation of intracellular Ca2+ downstream of PLCγ2. We have also demonstrated that this effect is, at least partially, mediated by the tyrosine kinase Pyk2. Pyk2 belongs to the focal adhesion kinase family and can be activated both by Src-mediated phosphorylation and by binding of Ca2+ to the N-terminal FERM domain.31,37 Pyk2 is highly expressed in platelets, and has been shown to be activated by many soluble agonists through both Ca2+-dependent and Ca2+-independent pathways.32-34 The role of Pyk2 in platelet function is still poorly characterized, and its involvement in integrin outside-in signaling has never been investigated directly. We have demonstrated herein that Pyk2 is activated after integrin α2β1 engagement and that this process requires PLCγ2 activity and intracellular Ca2+ increase, but not PI3Kβ. In contrast, we have clearly demonstrated that Pyk2 regulates PI3Kβ activity, because integrin α2β1–mediated phosphorylation of Akt was strongly impaired in Pyk2-deficient platelets. Generation of Pyk2-knockout mice allowed a better understanding of the role of this kinase in many physiologic context, including macrophage migration and osteoclast activation.27,38,39 In the present study, we report that Pyk2-deficient platelets show a defective activation of PI3Kβ after integrin α2β1–mediated adhesion. A possible role for Pyk2 in the regulation of platelet PI3K activity has been hypothesized previously on the basis of some circumstantial evidence.40,41 Our results definitively demonstrate that Pyk2 is an essential regulator of PI3Kβ in platelet integrin α2β1 signaling. We have been unable to document a direct association between Pyk2 and PI3Kβ in collagen-adherent platelets by coimmunoprecipitation experiments (data not shown). This evidence suggests either that the interaction occurs transiently and with a low affinity or that Pyk2-mediated stimulation of PI3Kβ involves an additional, as-yet-unidentified molecule. This possibility is currently under investigation. It should be noted, however, that whereas integrin-mediated PI3Kβ activation is totally suppressed in the absence of PLCγ2 or on chelation of intracellular Ca2+, it is reduced, but not abolished, in the absence of Pyk2. This implies that intracellular Ca2+ regulates PI3Kβ not only through activation of Pyk2, but also through other mechanisms that remain to be identified. In the present study, the analysis of Pyk2-knockout mice has been essential to demonstrate the role of this kinase in integrin signaling in mice, and we can simply assume, as in the case of many studies performed with other transgenic mice lacking specific signaling intermediates, that Pyk2 plays a similar role in humans. In the absence of any pathology associated with a deficiency of Pyk2, this assumption could only be confirmed pharmacologically. We have tested some commercially available Pyk2 inhibitors, but we have detected several nonspecific effects that precluded any further reliable investigation (data not shown).
In the present study, we have also investigated the role of the Pyk2/PI3Kβ pathway in platelet adhesion through integrin α2β1. We found that adhesion and spreading on monomeric collagen occurred normally in the absence of catalytically active PI3Kβ. We showed previously that adhesion and spreading on immobilized fibrinogen was severely compromised in PI3KβKD platelets.4 Therefore, it is clear that PI3Kβ plays different roles downstream of integrins α2β1 and αIIbβ3. This conclusion is not related to a difference in ligand density, which has been shown, for example, to affect outside-in signaling through integrin αIIbβ3,42,43 because we observed normal adhesion and spreading in the presence of PI3K inhibitors even after coating with a much lower concentration of monomeric collagen (eg, 0.1μg/mL; data not shown). Moreover, in agreement with the role of Pyk2 in PI3Kβ activation by integrin α2β1, we also found that platelets lacking Pyk2 were able to adhere normally and spread on monomeric collagen. It can be concluded that the previously reported role of PLCγ2 and intracellular Ca2+ on integrin α2β1–mediated spreading involves alternative signaling pathways. However, we were able to recognize a crucial role for Pyk2 and PI3Kβ in the inside-out activation of integrin αIIbβ3, allowing fibrinogen binding to collagen-adherent platelets. The defective integrin αIIbβ3 activation in the absence of the Pyk2/PI3Kβ pathway results in an impaired thrombus formation. Perfusion of whole blood on immobilized collagen under a moderate shear rate revealed that both Pyk2 and PI3Kβ are essential for correct thrombus formation. The stronger reduction of thrombus formation by PI3KβKD compared with Pyk2-knockout platelets is consistent with our observation that some residual activation of PI3Kβ still takes place in the absence of Pyk2. We showed previously that this cross-talk between the 2 main platelet integrins is dependent on PLCγ2 activity and is regulated by the small GTPase Rap1b.21,29 In the present study, we have shown that both PI3Kβ and Pyk2 are required for efficient stimulation of Rap1b. How PI3Kβ regulates binding of GTP to Rap1b is not clear. Activation of Rap1b by integrin α2β1 is completely dependent on the action of CalDAG-GEFI,21 and therefore it is likely that PI3Kβ can signal on CalDAG-GEFI. Although Ca2+ can stimulate CalDAG-GEFI, PI3Kβ signaling may be required as well. PI3Kβ also mediates Rap1b activation downstream of the P2Y12 ADP receptor.4,44 Although P2Y12 receptor is unable to increase intracellular Ca2+, activation of Rap1b by ADP is still dependent on CalDAG-GEFI.45 Our results actually support the model of a strict cooperation between Ca2+ and PI3Kβ for maximal activation of CalDAG-GEFI leading to GTP-Rap1b accumulation. Whatever the mechanism, it is clear that the small GTPase Rap1b integrates many signaling pathways initiated by PLCγ2 in integrin α2β1–adherent platelets and convey them to the inside-out activation of integrin αIIbβ3. A general scheme illustrating the role of PI3Kβ and Pyk2, the signaling pathway linking integrin α2β1 and integrin αIIbβ3, is depicted in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
In conclusion, our results identify a novel mechanism for PI3Kβ activation in integrin α2β1 outside-in signaling that depends on PLCγ2 and on the Ca2+-sensitive tyrosine kinase Pyk2, and suggest an important role for this pathway in the inside-out activation of integrin αIIbβ3.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Ministero dell'Istruzione, Università e Ricerca Scientifica (PRIN) and from Regione Lombardia and the University of Pavia (project REGLOM16 to I.C.). M.F. is supported by the British Heart Foundation (grant PG/06/022/20348).
Authorship
Contribution: A.C. and L.C. designed and performed the experiments and analyzed the data; G.G. and I.C. performed the experiments and analyzed the data; E.C. provided vital new reagents and performed the experiments; E.H., M.F., and M.O. provided vital new reagents and edited the manuscript; C.B. analyzed the data and edited the manuscript; and M.T. designed the research, analyzed the data, wrote the manuscript, and provided overall direction for the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mauro Torti, Department of Biochemistry, University of Pavia, via Bassi 21, 27100 Pavia, Italy; e-mail: mtorti@unipv.it.
References
Author notes
A.C. and L.C. contributed equally to this work.