To the editor:
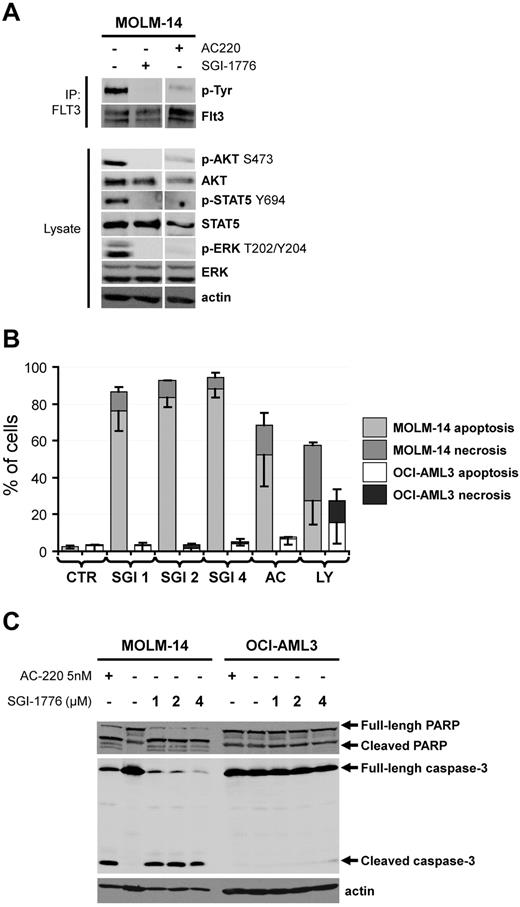
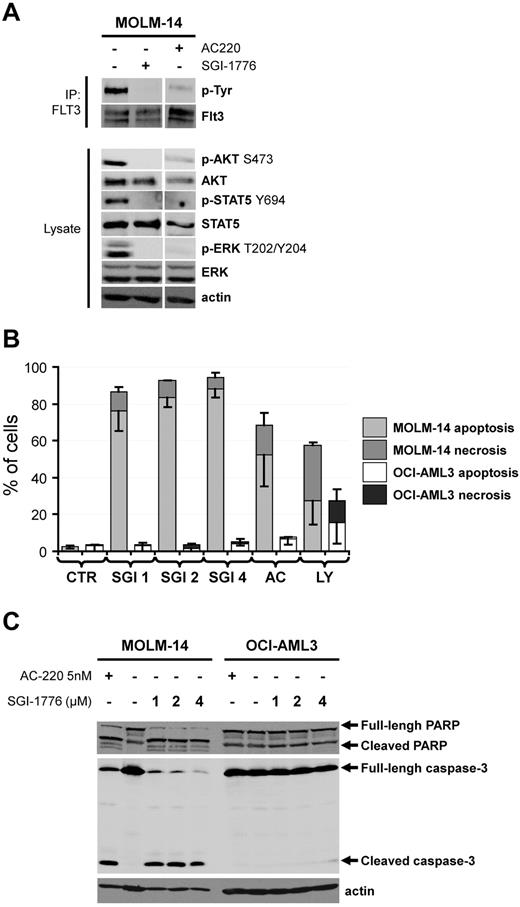
The Pim (for provirus integration site for Moloney murine leukemia virus) family of serine/threonine kinases are strongly involved in oncogenic processes and efforts are ongoing to develop specific Pim kinase inhibitors as anti-cancer therapy.1 Interestingly, recent reports from Gandhi's group emphasized the SGI-1776 compound (Astex Pharmaceuticals) as a potent Pim inhibitor with potential interest in chronic lymphocytic leukemia2 and in acute myeloid leukemia (AML).3 In this latter work, the SGI-1776 compound was showed to induce the apoptosis of both AML cell lines and primary AML samples, and to transiently reduce the size of tumors generated by subcutaneous injection of the MV4-11 human leukemic cell line without evidence for toxicity in mice.3 To explain the anti-leukemic activity of SGI-1776, the authors demonstrated an inhibition of Pim target phosphorylation such as c-Myc S62 and BAD S112. However, as SGI-1776 also inhibits the FLT3 kinase in vitro,2 major attention should be given to the FLT3 mutational status of AML cells, that is, the presence or not of FLT3 alleles harbouring internal tandem duplication (ITD). Chen and coworkers considered that SGI-1776 was effective against primary AML cells and AML cell lines regardless of their FLT3 mutational status; although a superior effect of this molecule was found in FLT3-ITD AML cells in some experiments.3 In contrast, we made slightly different observations. Although we also found an inhibition of Pim substrate phosphorylation in SGI-1776 treated AML cells (data not shown), we observed an inhibition of FLT3 autophosphorylation on tyrosine (Tyr) residues in the MOLM-14 FLT3-ITD AML cell line, which was similar to that observed after treatment with the specific FLT3 inhibitor AC-2204,5 (Figure 1A). Moreover, SGI-1776 inhibited the phosphorylation of well-known signaling relays downstream of FLT3, such as Akt S473, ERK T202/Y204 and STAT5 Y694, similarly to AC-220 (Figure 1A). Our results highly suggest that SGI-1776 directly inhibits the FLT3 kinase activity in AML. This conclusion could also explain the observation made by Chen and colleagues that SGI-1776 treatment results in an increased maturation of the FLT3-ITD in MV4-11 cells, assessed by an increased accumulation of the mature 150 kDa form compared with the 130 kDa endoplasmic reticulum form in SGI-1776–treated cells.3 Indeed, it has been previously demonstrated by Schmidt-Arras and coworkers that inhibition of FLT3-ITD catalytic activity promotes the maturation of the receptor with acquisition of complex glycosylation, leading to an increase of FLT3-ITD molecular mass from 130 to 150 kDa.6 We further showed that both AC-220 and SGI-1776 increased annexin V binding (Figure 1B) and induced the cleavage of caspase-3 (Figure 1C) in the MOLM-14 (FLT3-ITD) but not in the OCI-AML3 (FLT3-WT) AML cell lines. Although Chen and colleagues observed apoptosis in SGI-1776–treated OCI-AML3 cells, higher doses of SGI-1776 were required in their experiments to induce apoptosis in these cells expressing FLT3-WT than in MV4-11 or MOLM-13 cells that express FLT3-ITD receptors. Similarly, higher doses of SGI-1776 were required to inhibit Pim target phosphorylation or RNA synthesis.3 Thus, our explanation is that the inhibitory effects of SGI-1776 reported by Chen and colleagues are mostly because of the inhibition of FLT3 catalytic activity. Overall, we assume that no definitive conclusion on the role of the Pim kinases in the survival of AML cells could be established as yet.
The SGI-1776 compound is a FLT3 kinase inhibitor and preferentially induces apoptosis in AML cells harboring a FLT3-IT3 mutation. (A) The FLT3-ITD positive AML cell line MOLM-14 was treated or not with 4μM SGI-1776 or 5nmol/l AC-220 during 1 hour. The FLT3 tyrosine autophosphorylation was then evaluated by FLT3 immunoprecipitation (IP) revealed with an anti–phospho-tyrosine (p-Tyr) antibody as reported.7 Whole cell lysates were submitted to Western blot analysis using anti–phospho-Akt S473, ERK Y202/Y204 and STAT5 Y694 antibodies and anti-Akt, ERK, STAT5 and actin antibodies, as reported.8 (B) The MOLM-14 (FLT3-ITD) and OCI-AML3 (FLT3-WT) AML cell lines were cultured 48 hours without (CTR histograms) or with 1, 2 or 4μM SGI-1776 (SGI 1, 2 and 4 histograms, respectively), 5nmol/l AC-220 (AC histograms. AC-220 is a specific FLT3 inhibitor) or 25μM LY294002 (LY histograms. LY294002 is a broad-spectrum serine/threonine kinase inhibitor9 ). Apoptosis and necrosis were determined by Flow Cytometry as annexin V positive and 7AAD negative and annexin V positive and 7AAD positive AML cells, respectively. (C) The MOLM-14 and OCI-AML3 AML cell lines were cultured 48 hours without or with 1, 2 or 4μM SGI-1776 or 5nmol/l AC-220 and cell lysates were submitted to Western blot using anti-PARP, anti–caspase-3 and anti-actin antibodies.
The SGI-1776 compound is a FLT3 kinase inhibitor and preferentially induces apoptosis in AML cells harboring a FLT3-IT3 mutation. (A) The FLT3-ITD positive AML cell line MOLM-14 was treated or not with 4μM SGI-1776 or 5nmol/l AC-220 during 1 hour. The FLT3 tyrosine autophosphorylation was then evaluated by FLT3 immunoprecipitation (IP) revealed with an anti–phospho-tyrosine (p-Tyr) antibody as reported.7 Whole cell lysates were submitted to Western blot analysis using anti–phospho-Akt S473, ERK Y202/Y204 and STAT5 Y694 antibodies and anti-Akt, ERK, STAT5 and actin antibodies, as reported.8 (B) The MOLM-14 (FLT3-ITD) and OCI-AML3 (FLT3-WT) AML cell lines were cultured 48 hours without (CTR histograms) or with 1, 2 or 4μM SGI-1776 (SGI 1, 2 and 4 histograms, respectively), 5nmol/l AC-220 (AC histograms. AC-220 is a specific FLT3 inhibitor) or 25μM LY294002 (LY histograms. LY294002 is a broad-spectrum serine/threonine kinase inhibitor9 ). Apoptosis and necrosis were determined by Flow Cytometry as annexin V positive and 7AAD negative and annexin V positive and 7AAD positive AML cells, respectively. (C) The MOLM-14 and OCI-AML3 AML cell lines were cultured 48 hours without or with 1, 2 or 4μM SGI-1776 or 5nmol/l AC-220 and cell lysates were submitted to Western blot using anti-PARP, anti–caspase-3 and anti-actin antibodies.
Authorship
Acknowledgments: The authors thank all participating investigators from the GOELAMS. They thank Dr Pietro Taverna for providing them the SGI-1776 compound.
This work was supported by grants from the Ligue Nationale Contre le Cancer (LNCC, comité d'Ile de France), the Fondation de France (comité leucémies), the Institut National du Cancer (INCa), and the Association Laurette Fugain.
Contribution: M.A.H and A.S.G performed research and analyzed data; C.L and P.M analyzed data; D.B and J.T designed research and analyzed data; J.T. wrote the letter; and all authors read and contributed to the final version of the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jerome Tamburini, Institut Cochin, Departement of Hematology-Immunology, 22 rue Méchain, 75014 Paris, France; e-mail: jerome.tamburini@inserm.fr.
References
Author notes
M.-A.H. and A.S.G. contributed equally to this work.