Abstract
Macrophage-colony stimulating factor (CSF-1) signaling through its receptor (CSF-1R) promotes the differentiation of myeloid progenitors into heterogeneous populations of monocytes, macrophages, dendritic cells, and bone-resorbing osteoclasts. In the periphery, CSF-1 regulates the migration, proliferation, function, and survival of macrophages, which function at multiple levels within the innate and adaptive immune systems. Macrophage populations elicited by CSF-1 are associated with, and exacerbate, a broad spectrum of pathologies, including cancer, inflammation, and bone disease. Conversely, macrophages can also contribute to immunosuppression, disease resolution, and tissue repair. Recombinant CSF-1, antibodies against the ligand and the receptor, and specific inhibitors of CSF-1R kinase activity have been each been tested in a range of animal models and in some cases, in patients. This review examines the potential clinical uses of modulators of the CSF-1/CSF-1R system. We conclude that CSF-1 promotes a resident-type macrophage phenotype. As a treatment, CSF-1 has therapeutic potential in tissue repair. Conversely, inhibition of CSF-1R is unlikely to be effective in inflammatory disease but may have utility in cancer.
Introduction
Cells of the mononuclear phagocyte system contribute to the pathology of major diseases and at the same time are essential for normal development, innate and acquired immunity, homeostasis, and tissue repair.1-6 They enter the circulation from the marrow as monocytes and leave the blood in response to a wide range of signals to either contribute to inflammatory processes or take up residence in specific locations in tissues.3,5 The function of macrophages in inflammation varies depending on the nature of the stimulus. Broadly speaking, 2 major pathways of macrophage activation have been described associated with the activation of distinct T lymphocyte immune responses. Classical activation is associated with the actions of IFN-γ and is directed toward killing of microbial pathogens.7 Alternative activation, involving responses to IL-4 or IL-13, has been associated with parasitic and allergic diseases.1 These states of macrophage activation have also been called M1 and M2, respectively, linked to the activation of Th1 and Th2 cells, and are considered mutually exclusive and antagonistic. M2 macrophages have also been ascribed functions in immunosuppression and vascularization in tumors.8,9 Biswas and Matonavani have extended the classification into further subclasses of M2-like macrophages.10 Markers of the different activation states have been proposed. For example, in the mouse, Arg1, Fizz, and Ym1 (chi3l3) have been considered M2 markers, whereas elevated MHC class II, CD86, and iNOS expression are associated with M1 polarization.1,7 However, the markers do not correlate very well with each other when analyzed across large datasets or in responses to different stimuli.1,11 In reality, each pathogen and each pathology probably generates a unique macrophage phenotype that also varies with time from the onset to the resolution (or chronic progression) of the response. Within macrophage populations, individual cells may also be infinitely heterogeneous because of the stochastic nature of transcription control.12 As Mosser and Edwards13 suggest, it is more appropriate to see macrophage heterogeneity in terms of the diversity of points on a color wheel, rather than on a linear scale between M1 and M2, or alternative and classical extremes. Within such a spectrum, it is also debatable whether the antigen-presenting dendritic cell (DC) can be considered as a separate entity distinguishable by function or markers from macrophages.4,14
One essential regulator of macrophage homeostasis in vivo is macrophage colony-stimulating factor, or CSF-1, so named because it was the first of the hemopoietic growth factors to be isolated as a pure protein and because it can promote the growth of pure colonies of macrophages from bone marrow progenitors in semisolid media in vitro.15 Although CSF-1 has this activity, it is not the only factor that can promote macrophage growth from marrow cells, and indeed the numbers of colonies and their size are greatly increased when it acts in combination with other factors, including GM-CSF, IL-3, and IFN-γ.16-18 Nevertheless, natural mutations of the Csf-1 locus in mouse (op/op) and rat (tl/tl) confirmed that CSF-1 has a nonredundant function in controlling macrophage numbers in tissues and additionally revealed numerous pleiotropic consequences of CSF-1 deficiency, including severe growth retardation and low fertility.6,19,20 The osteopetrosis seen in CSF-1–deficient animals is a consequence of deficient production of bone-resorbing osteoclasts, which share a progenitor with macrophages and express the CSF-1 receptor.19,21,22
Human CSF-1 cDNA was cloned independently by 2 groups in the late 1980s.23,24 We now recognize that there are actually 3 major forms of CSF-1 protein produced from alternatively spliced transcripts: the predominant secreted proteoglycan, a secreted glycoprotein, and cell-surface membrane-anchored form that can be released by proteolytic cleavage.19 Each shares an N-terminal region containing an active 149-amino acid fragment that forms a 4-helix bundle. The crystal structure of the active fragment was solved by Pandit et al.25 CSF-1 acts on its target cells by binding to CSF-1R (c-fms), a member of the type III protein tyrosine kinase receptor family. The crystal structure of the CSF-1/CSF-1R complex was described by Chen et al.26 Recently, a second ligand for the CSF-1R, IL-34, was described,27 potentially explaining some of the differences in severity between the op/op mouse and a CSF-1R mutation.28 The spatiotemporal expression of IL-34 differs from that of CSF-1, suggesting that they have distinct biologic functions.29 The two ligands acting on the same receptor are conserved across evolution to birds. CSF-1 and the binding sites on the receptor have evolved rapidly across species. By contrast, IL-34 is much more conserved across species, and computational modeling suggests that it binds to different parts of the receptor.30 In keeping with this view, Chihara et al identified mAbs binding to CSF-1R that can block CSF-1, but not IL-34 binding.31 The two proteins induced equivalent expression of chemokine genes when added to human whole blood,32 but Chihara et al found subtle difference in signal intensity between the two ligands on mouse cells.31 The very high level of conservation of IL-34 across species is atypical of immune-associated genes and could reflect an essential function. Thus far, no viable mouse knockout of IL-34 has been reported. If the knockout is lethal, where the CSF-1R knockout is known to be viable on some backgrounds,28 this would imply the existence of another receptor for IL-34. That, in turn, would have implications for the use of CSF-1R antagonists, which could lead to elevated IL-34 levels acting on an alternative target. The mRNA encoding the CSF-1R is expressed in a highly restricted manner in macrophage lineage cells in both mouse and human (www.biogps.gnf.org) and from a separate promoter (which differs between mouse and human33 ) in placental trophoblasts and in osteoclasts.34 The promoter region of the mouse locus has been characterized and used to produce a CSF-1R-EGFP transgenic mouse line, which enables the visualization of macrophages in tissues.35
Shortly after recombinant CSF-1 became available, its ability to expand the mononuclear phagocyte system after administration in vivo was demonstrated in mice36 and subsequently in rats,37 nonhuman primates,38 and humans.39 Increased levels of circulating CSF-1 were detected in many different human disease states or animal models. Pharmacologic disruption of the CSF-1/CSF-1R axis to modulate macrophage populations has therapeutic potential in 4 broad clinical settings: inflammatory disease, cancer, autoimmunity, and bone disease (Figure 1). Accordingly, many different companies have explored different approaches to blocking CSF-1 action. In this review, we critically review animal and human studies of CSF-1 biology and explore the potential applications of both CSF-1 and CSF-1R antagonists in human medicine.
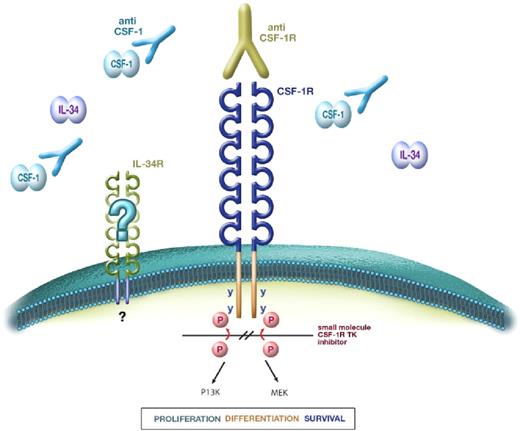
CSF-1 receptor signaling and blockade strategies. CSF-1 and IL-34 bind to the extracellular domain of the CSF-1R to induce dimerization and tyrosine kinase (TK)–mediated autophosphorylation of cytoplasmic tyrosine residues, leading to a cascade of intracellular signals, which regulate the production, survival, and function of macrophages. To date, no alternative receptor for IL-34 has been identified. Disruption of the CSF-1/CSF-1R axis can be achieved using neutralizing anti–CSF-1 mAbs or anti–CSF-1R mAbs (the latter can block binding of either CSF-1 or IL-34 or both cytokines) or inhibition of CSF-1R tyrosine kinase using small-molecule TK inhibitors.
CSF-1 receptor signaling and blockade strategies. CSF-1 and IL-34 bind to the extracellular domain of the CSF-1R to induce dimerization and tyrosine kinase (TK)–mediated autophosphorylation of cytoplasmic tyrosine residues, leading to a cascade of intracellular signals, which regulate the production, survival, and function of macrophages. To date, no alternative receptor for IL-34 has been identified. Disruption of the CSF-1/CSF-1R axis can be achieved using neutralizing anti–CSF-1 mAbs or anti–CSF-1R mAbs (the latter can block binding of either CSF-1 or IL-34 or both cytokines) or inhibition of CSF-1R tyrosine kinase using small-molecule TK inhibitors.
Control of circulating CSF-1
CSF-1 is present in the circulation, predominantly as the proteoglycan form, at biologically active concentrations of approximately 10 ng/mL. It is produced constitutively by a wide variety of cells of mesenchymal and epithelial origin.6,19 The level in the circulation increases in many different pathologies, including infections, cancer, and chronic inflammatory disease, regardless of etiology.19,20,40 CSF-1 levels are also elevated in the circulation during pregnancy and contribute to placentation.41,42 In both mice and humans, there is a perinatal surge of tissue and circulating CSF-1.43,44 In inflammation, CSF-1 may also be produced by recruited macrophages themselves, although in the mouse at least, most macrophages do not produce CSF-1 and undergo cell death in the absence of the protein.45,46 Under normal steady-state conditions, the production of CSF-1 is balanced by its consumption by tissue macrophages, through receptor-mediated endocytosis by the CSF-1R followed by intracellular destruction.47
Effects of CSF-1 in vitro
Biologic effects of CSF-1 and the signaling pathways from the receptor have been reviewed in detail elsewhere.19 Addition of CSF-1 in cell culture can accelerate differentiation and maturation of monocytes into active phagocytes. In humans, CSF-1 is commonly used to generate monocyte-derived macrophages in vitro,48 and this process has been analyzed using cDNA microarrays.49 The phenotype of these monocyte-derived macrophages has been contrasted to the cells generated when monocytes are cultured in GM-CSF to produce monocyte-derived DCs, which have an increased capacity for antigen presentation.50 A meta-analysis of mouse microarray data indicates that CSF-1– and GM-CSF–stimulated cells are more similar than different, and both are clearly phagocytes.11 Nevertheless, CSF-1 as the sole stimulus polarizes macrophages away from an antigen-presenting phenotype and toward an immunosuppressive function in both mouse and human.4,14 Hence, macrophages generated in response to CSF-1 have been proposed by some to be alternatively activated or M2-like.49,51 Because CSF-1–stimulated macrophages can still respond to the M2-inducing lymphokine IL-4 with significant changes in gene expression,1,49,51,52 it is more appropriate to consider the CSF-1–stimulated cell as a distinct entity. One of the most studied and unique targets of CSF-1 signaling in mice and humans is the proteolytic enzyme urokinase plasminogen activator,53,54 a target of the ras-raf-MAPK pathway,55 which contributes to regulated fibrinolytic activity. But caution is needed when extrapolating from mouse experiments on CSF-1. In humans, but not in mice, continuous exposure to CSF-1 appears to drive a proatherogenic phenotype,48 in keeping with an inferred role in atherosclerosis.56 Broadly speaking, based on in vitro data, CSF-1 as a sole stimulus might be expected to generate actively phagocytic macrophages that promote extracellular proteolysis and tissue repair and suppress cell-mediated immunity.
Effects of CSF-1 administration in vivo
The simple homeostatic mechanism that balances macrophage numbers with production and receptor-mediated clearance of CSF-147 is clearly disturbed when exogenous CSF-1 is administered, receptor-mediated clearance is saturated, and the concentration in the circulation rises. The administration of CSF-1 can generate a transient increase in c-fos mRNA in the spleen, providing a simple in vivo bioassay and suggesting that CSF-1 availability is not saturating for tissue macrophages.57 To generate a more sustained response leading to measurable changes in macrophage numbers, the molecular size of CSF-1 becomes critical because macrophages and their progenitors require continued exposure to CSF-1 to enter the cell cycle.58 The unglycosylated minimal active fragment of CSF-1, a disulphide-linked homodimer produced in bacteria by Chiron, is cleared rapidly by the kidney with a half-life of 1 to 2 hours.59 The recombinant protein produced by Genetics Institute was made in mammalian cells and was a secreted 70- to 90-kDa glycosylated protein, with a longer C terminus, closely resembling the form that can be isolated from human urine.24 In consequence, the larger protein has a much slower rate of renal clearance and a 5-fold lower dose of the mammalian-expressed protein (0.5-1 mg/kg per day) than the bacterial recombinant protein (4 mg/kg per day), was required to generate substantial responses in mice.
The first demonstrated efficacy of recombinant human CSF-1 in mice showed that treatment each day for 4 days caused a 10-fold increase in blood monocyte numbers.36 More recently, CSF-1 was shown to increase conventional and plasmacytoid DC numbers.60,61 The latter finding is consistent with the expression of CSF-1R (CD115) on the shared macrophage/DC progenitor cell and on mature DCs themselves.61,62 Prolonged CSF-1 administration to mice also altered osteoclastic function and generated an increase in serum markers of bone turnover.22 There was no net change in bone mineral density, presumably because of the coupling of osteoclast and osteoblast function. Indeed, there was an unexpected and unexplained increase in the trabecular bone, which would suggest the possible use of CSF-1 as an anabolic agent.
The maximal response to CSF-1 apparently required the repeated administration.36 Ulich et al conducted a single dose escalation study in rats, and a dose of 0.5 to 1 mg/kg caused a transient spike in monocyte count of approximately 5- to 10-fold after 24 hours, which returned to baseline by 36 hours.37 Prolonged treatment led to maintenance of the elevated monocyte count, and the only apparent toxicity was a thrombocytopenia. Subsequent studies progressed to rabbits and nonhuman primates38,63 and used continuous intravenous infusion or twice-daily subcutaneous injection. Repeated infusions after 2 weeks generated comparable increases in monocyte count. The same group subsequently noted a substantial decrease in plasma cholesterol in the treated animals63 ; precisely how this is related to the up-regulation of cholesterol biosynthesis in human monocyte-macrophages by CSF-148 is unclear.
The role of CSF-1 in monocyte maturation
This initial study of treatment of mice with CSF-1 did not distinguish among subpopulations of monocytes. Blood monocytes in mouse and human are heterogeneous in terms of surface markers. In humans, two populations have been distinguished based on the level of expression of the Fc receptor, CD16, which varies inversely with that of the lipopolysaccharide coreceptor, CD14.64,65 Similarly in mice, the expression of Ly6C and the fractalkine receptor CX3CR1 varies inversely to distinguish two subpopulations that are thought to be functionally equivalent to the human populations.64,66 One monocyte population (CD14 (hi) or Ly6C (hi) in human and mouse, respectively) is short-lived in the circulation and recruited in response to inflammatory stimuli, notably in response to chemokines that interact with the receptor CCR2.64,66,67 A recent paper from a consortium of investigators suggested these be called “classical” rather than “inflammatory” monocytes.65 By contrast, the precursors of resident tissue macrophages, subsets of which have also been reported to patrol vessel walls,68 were referred to as “nonclassical.” There was also an “intermediate” population identified. These designations are rather artificial; subdivision seems to require a rather arbitrary assignment of the location of gates on a flow cytometer. As discussed below in the context of anti–CSF-1R treatments, there is evidence that the monocyte “subpopulations” are actually a maturation series controlled by CSF-1.65,69 Accordingly, where CSF-1 caused a 5-fold increase in blood monocytes in nonhuman primates, the large majority expressed the “nonclassical” maturation marker, CD16.38 Furthermore, in keeping with the role of “nonclassical” cells as precursors of tissue macrophages, CSF-1 treatment of mice also caused a very large increase in resident tissue macrophage numbers and in expression of the maturation marker, F4/80.36
Early preclinical and clinical studies of CSF-1
CSF-1 has had limited application as a hemopoietic growth factor in the setting of bone marrow transplantation or during recovery from myelosuppressive chemotherapy, where other myeloid growth factors, such as G-CSF and GM-CSF, are in common use.70 One reported phase 3 trial by Masaoka et al, using purified CSF-1 from human urine, reported a reduction in circulating granulocyte recovery time and improved survival without retransplantation in marrow transplant patients.71 Similarly, Hidaka et al reported the use of CSF-1 (also known as Mirimostim) in patients with severe neutropenia after chemotherapy for ovarian cancer.72 Their data suggested an improvement in NK-cell function and numbers and T-cell maturation as well as an improvement in granulocyte function that was not improved by combination with G-CSF. Arguably, there is a rationale for exploring the efficacy of combined treatments CSF-1 and GM-CSF. In the mouse, the two factors act together on high proliferative potential precursors to generate very large colonies.16,17 In humans, very low concentrations of GM-CSF were required to generate large macrophage colonies in standard colony assays using CSF-1.18
Phase 1 clinical trials of recombinant CSF-1 (produced by Genetics Institute) by continuous infusion in humans with advanced melanoma confirmed the ability to increase circulating monocyte numbers.73,74 The increases reversed rapidly on cessation of infusion and were reproduced on reinfusion. The transient thrombocytopenia and the decrease in circulating cholesterol seen in the previous animal studies were confirmed in these patients. The choice of melanoma as target for such trials was based in part on animal studies in which CSF-1 prevented metastasis of the B16 melanoma75 and evidence for the ability of CSF-1–stimulated cells to kill melanoma cells in vitro.38 Although there was anecdotal evidence of efficacy in a small number of the treated patients in these trials, to our knowledge there has not been a follow-up trial. A separate phase 1 study in cancer patients using rhCSF-1 produced by Chiron also observed mild thrombocytopenia in some persons and also unexplained hyperglycemia.76 This study did not identify any therapeutic effect on the cancers.
The other initial target for CSF-1 therapy was fungal diseases, where CSF-1–stimulated macrophages were purported to phagocytose organisms, such as Candida.77 There was no reported follow-up from the initial clinical studies in humans that suggested an increase in survival rate of bone marrow transplant patients with disseminated candidiasis78 ; it is not known whether this was the result of a lack of commercial interest or a lack of reproducibility. Around the time of these studies, it became clear that CSF-1 treatment could exacerbate the pathology of certain diseases in animal infectious and inflammatory disease models, not surprisingly, because pathology can be macrophage-mediated.79 Several studies have explored the role of CSF-1 in the pathology of autommune nephritis, combining studies of ectopic CSF-1 with increased or reduced CSF-1 levels in the op/op or transgenic mice overexpressing various forms of the protein.80-82 These studies indicated that CSF-1 drives increased production of inflammatory or “classical” macrophages from the marrow and also local proliferation within the kidney.83 Indeed, contrasting studies of candidiasis were informative. Whereas pretreatment of animals with CSF-1 was found to be protective,77 treatment of animals with an established disseminated candidiasis accelerated the disease.77,84 On initial exposure, CSF-1–stimulated macrophages are able to recognize and clear the pathogen. Once the disease is established, there is massive macrophage infiltration of the affected organs (the kidneys); these cells produce inflammatory cytokines that mediate the pathology. Accordingly, the CSF-1 treatment drastically accelerated weight loss in the Candida-infected mice.84
Recent advances in the applications of CSF-1
The potential importance of CSF-1 in the regulatory balance of antigen presentation is supported by recent evidence of csf1r gene polymorphisms associated with altered CSF-1R expression being linked to asthma susceptibility.85 The gene encoding the ligand, CSF-1, also varies between persons and is strongly linked to Paget disease susceptibility.86 The ability of macrophages in certain activation states to suppress T-cell responses and to elicit tolerance has been known for a very long time.14,87 As noted in “Effect of CSF-1 in vitro,” in both mice and humans, populations of macrophages polarized with CSF-1 as the sole stimulus are generally relatively poor at stimulating T cells in vitro. In vivo, the F4/80 antigen, which is inducible by CSF-1,36 increased on maturation, and generally considered a macrophage marker,3,4 is required for the generation of oral tolerance. Furthermore, F4/80 KO mice are deficient in regulatory T cells (Tregs).88 An F4/80+ macrophage population, which expresses the transcription factor Foxp3, normally expressed by Treg, was recently identified and apparently suppressed T-cell responses and induce Treg conversion in vivo.89 This work was subsequently retracted. Nevertheless, in mice, treatment with CSF-1 has been shown to cause profound immunosuppression; T cells from the spleens of treated animals were unresponsive to T-cell mitogens or allogeneic cells.90 CSF-1 was also shown to directly suppress allogeneic T-cell responses in vitro91 and cutaneous hypersensitivity responses in vivo.92
CSF-1 was reported to be substantially elevated in the circulation in acute GVHD in mice.93 Blazar et al were the first to assess the impact of CSF-1 treatment in bone marrow transplantation, examining T cell-depleted grafts, with and without a potential NK-cell contribution to their recognition by the recipient.94 They found an inhibitory effect of CSF-1 on engraftment, but only if the recipient NK cells were able to recognize and eliminate the graft. Indeed, others had reported that CSF-1 treatment led to increases in the numbers of NK cells and their mobilization from the marrow by an unknown mechanism.95 More recently, the effect of recipient CSF-1 pretreatment was investigated in an acute GVHD model after transfer of BALB/c bone marrow and spleen cells into irradiated C57Bl/6 recipients.96 Pretreatment reduced the severity of disease without apparently altering engraftment. These data were used to argue for distinct functions of “macrophages” versus “DCs” in GVHD pathology. We have pretreated B6D2F1 recipients for 4 days with CSF-1 at a 2× higher dose than used by Hashimoto et al,96 before irradiation and transplantation of parental B6 bone marrow supplemented with splenic T cells. Although we saw expansion of both macrophages and CD11c-positive cells in the recipient mice as expected from previous studies,36,61 the pretreatment had no effect on survival or GVHD severity (K.P.A.M. and S. Oliver, unpublished data, May 2008). Similarly, in a CD8 T cell–dependent model (C3H.SW into B6), we also found no effect of pretreatment (K.P.A.M. and S. Oliver, unpublished data, January 2009). We can only conclude that a limiting role for macrophage-mediated removal of alloreactive T cells is a specific feature of the model or strain combination used by Hashimoto et al.96 Pretreatment of a human bone marrow transplant recipient with CSF-1 has not been reported, and the impact is difficult to predict from the mouse studies, which are specifically designed to generate a substantial GVH response. There is, thus far, no reproducible evidence of clinical utility for CSF-1 as a treatment to suppress acute GVHD. Two separate studies in Japan71,97 and one in the United States98 found that CSF-1 (M-CSF) treatment had no effect on acute GVHD or relapse rate. The former study reported a small impact on extensive chronic GVHD.
The more promising trend in the recent studies of CSF-1 as a therapeutic agent relates to its trophic actions.6,21 Building on the evidence of the role of CSF-1 in reproduction and pregnancy mentioned above, and initial data on rats,99 Nishimura et al found a correlation between elevated CSF-1 in the serum and successful ovulation induction in an in vitro fertilization program.100 They subsequently reported that coadministration of CSF-1 with gonadotrophins could increase the numbers of oocytes, fertilized eggs, and transferred embryos101
The pro-repair phenotype noted in “Effects of CSF-1 in vitro” includes the expression of numerous growth factors for other cells (eg, IGF-1, PDGFB, and VEGFA), endocytic receptors (eg, macrophage scavenger receptors), and proteolytic enzymes in addition to urokinase plasminogen activator.6,19-21 CSF-1 probably contributes to the local macrophage proliferation that is one hallmark of Th2-driven inflammation,102 especially because mouse inflammatory macrophages recruited by a sterile stimulus can be autocrine for CSF-1.45
Among the systems that have been studied are fracture repair and macrophage-osteoblast interactions,103 repair after ischemia in the kidney104,105 and heart,106 promotion of angiogenesis,107 and elimination of amyloid deposits in the brain.108,109 Two biologic issues arise from these studies. First, there is the question of whether CSF-1–stimulated macrophages can interconvert into cells of mesenchymal lineages. We found some evidence using a CSF-1r–EGFP transgenic reporter that myeloid cells can give rise to myofibroblasts.110 In the case of angiogenesis, there is some evidence for bone marrow precursors of endothelial cells. Monocytes can apparently transdifferentiate into endothelium in vitro, but in vivo the major effect of CSF-1 is to promote production of proangiogenic growth factors.107 The second issue is whether the CSF-1R is expressed on cells outside of the mononuclear phagocyte lineage. Menke et al found that CSF-1 treatment can promote repair in a renal ischemia reperfusion model in mice and suggested that the effects were partly the result of direct effects on the tubular epithelial cells, which they claimed expressed significant levels of CSF-1R in response to the insult.105 Jose et al saw no evidence of CSF-1R protein expression by renal epithelial cells in rejecting renal allografts, where the ligand CSF-1 was also expressed very highly by tubular cells.83 According to Menke et al, a nonmacrophage-mediated mechanism of CSF-1 action was supported by depleting the animals of CD11b-positive cells using a CD11b-DTR transgene,105 but these authors did not apparently detect the resident macrophages, which are actually extremely numerous in the kidney and do not express CD11b.111 Macrophages may be a significant contaminant of primary renal epithelial cell cultures as they are in cultured calvarial osteoblasts; thus, contaminating macrophages could explain the apparent expression of CSF-1R.112 Alikhan et al recently published the same basic observation: that CSF-1 can promote renal repair after reversible ischemia.104 But using the same CSF-1R–EGFP transgene, they did not see any evidence of induced CSF-1R expression by tubular epithelial cells and instead demonstrated that the treatment-increased macrophage recruitment, polarized the cells toward an M2-like phenotype and promoted production of growth factors, such as IGF1. That finding built on an earlier observation: that CSF-1 can promote the growth of embryonic kidney in tissue culture,113 again a system in which there was no evidence of expression of CSF-1R in the renal parenchyma outside of the macrophages.
Blocking the actions of CSF-1
There are two approaches to blocking the action of CSF-1: the use of inhibitors directed against the protein tyrosine kinase activity of the receptor and the use of agents that block the binding of CSF-1 to its receptor (Figure 2). The latter category includes antibodies against the receptor, antibodies against the ligand, and soluble receptors. There are several complexities that arise from the use of CSF-1R inhibitors that are commonly neglected.
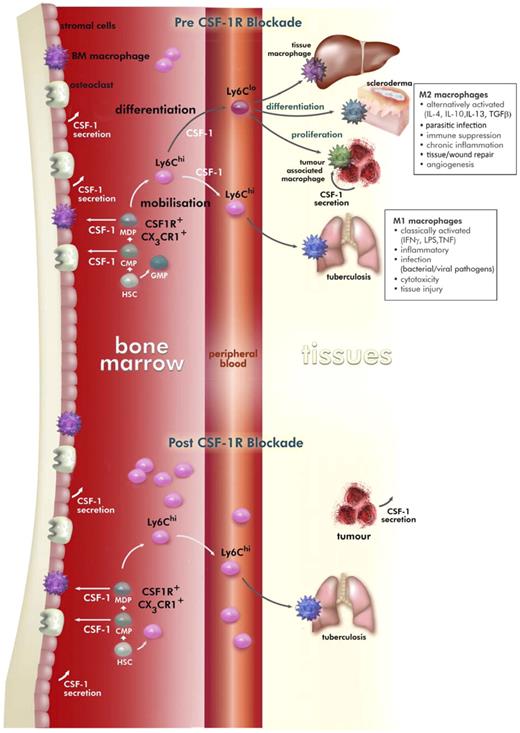
CSF-1 regulation of macrophage development in the mouse and the effects of prolonged M279 anti–CSF-1R mAb treatment. HSCs give rise to common myeloid precursors, which generate monocyte-DC precursors that in steady state give rise to DC precursors and CSF-1R+Ly6Chi and CSF-1R+Ly6Clo monocytes. Monocytes enter the circulation from the marrow and are signaled to exit the blood to contribute to inflammatory processes (CSF-1R+Ly6Chi) or to take up residence in specific locations (CSF-1R+Ly6Clo). CSF-1 is a critical regulator of the differentiation, proliferation, and survival of CSF-1R+Ly6Clo monocyte-derived tissue macrophages, alternatively activated macrophages, and tumor-associated macrophages. The cellular sources of CSF-1 are primarily mesenchymal in origin, but macrophages and tumors can also secrete this cytokine. Treatment with CSF1R blocking antibody M279 selectively depleted the CSF-1R+Ly6Clo monocyte precursor of resident tissue macrophages, whereas CSF-1R+Ly6Chi inflammatory monocytes were increased. Within tissue, the M279 mAb may prevent differentiation and proliferation of resident macrophage populations and tumor-associated macrophages.
CSF-1 regulation of macrophage development in the mouse and the effects of prolonged M279 anti–CSF-1R mAb treatment. HSCs give rise to common myeloid precursors, which generate monocyte-DC precursors that in steady state give rise to DC precursors and CSF-1R+Ly6Chi and CSF-1R+Ly6Clo monocytes. Monocytes enter the circulation from the marrow and are signaled to exit the blood to contribute to inflammatory processes (CSF-1R+Ly6Chi) or to take up residence in specific locations (CSF-1R+Ly6Clo). CSF-1 is a critical regulator of the differentiation, proliferation, and survival of CSF-1R+Ly6Clo monocyte-derived tissue macrophages, alternatively activated macrophages, and tumor-associated macrophages. The cellular sources of CSF-1 are primarily mesenchymal in origin, but macrophages and tumors can also secrete this cytokine. Treatment with CSF1R blocking antibody M279 selectively depleted the CSF-1R+Ly6Clo monocyte precursor of resident tissue macrophages, whereas CSF-1R+Ly6Chi inflammatory monocytes were increased. Within tissue, the M279 mAb may prevent differentiation and proliferation of resident macrophage populations and tumor-associated macrophages.
CSF-1R is part of a family of receptors, the intracellular domains of which are very closely related. Of the many available inhibitors,114 GW2580 has the greatest apparent specificity for CSF-1R versus related kinases, such as c-kit and Flt3.115 This inhibitor does not inhibit the avian CSF-1R,30 so specificity is not solely determined by the ATP-binding pocket (and the inhibitor is therefore more likely to be truly specific). But in vitro assays are not entirely predictive of the activity in whole cells.45
With the discovery of a second ligand for the CSF-1 receptor, IL-34, there is the question for antibodies of whether they inhibit both of the ligands.27,30 Both anti–mouse CSF-1R antibodies described in the paragraphs below inhibit both CSF-1 and IL-34 actions29 (and D.A.H. and L. Bonham, unpublished data, September 2006), although as noted earlier, the two ligands probably bind different parts of the receptor, and selective monoclonals have also been described.31
Because CSF-1 is cleared from the circulation by receptor-mediated endocytosis,47 CSF-1R blockade causes a massive elevation of circulating CSF-1 concentration. As the antibody concentration subsides, there is a consequential enhanced CSF-1 signal and rebound monocytopoiesis. This will also occur if CSF-1R–bearing cells are killed, for example, by toxic liposomes or with macrophage-directed toxic transgenes.116 However, it does not occur when CSF-1R kinase inhibitors are added, unless they are toxic, because receptor-mediated internalization of CSF-1 does not require the kinase activity of the receptor.45
There has been relatively little use made of anti–CSF-1 antibodies. Wei et al generated a neutralizing rabbit anti-serum and examined the effect of PEGylated-antibody on postnatal development.117 They essentially recapitulated many of the growth retardation and developmental abnormalities of the op/op mouse, supporting the view that the postnatal surge of CSF-1 is important for organ maturation and somatic growth.6,21 Lokeshwar and Lin generated a rat anti–CSF-1 neutralizing mAb,118 which has been used to demonstrate a role for CSF-1 in inflammation and joint erosion in collagen-induced arthritis.119 The 5A1 mAb was also used in ovariectomized mice to demonstrate CSF-1 dependence in estrogen deficiency-induced bone loss,120 raising the possibility that such therapy could be applied in human osteoporosis. Anti–CSF-1 mAb has been tested in nonhuman primates,121 generating a selective depletion of the “mature” or resident CD16+ monocyte population and a time-dependent loss of Kupffer cells from the liver. These findings support the role of CSF-1 in monocyte maturation discussed in “The role of CSF-1 in monocyte maturation.” A phase 1/2 clinical trial of anti–CSF-1 antibody in prostate cancer with bone metastasis, to reduce osteolytic activity, was commenced by Novartis but discontinued for strategic reasons (http://clinicaltrials.gov/ct2/show/NCT00757757).
The first neutralizing mAb directed against CSF-1R, AFS98, was produced by Sudo et al, who used it to demonstrate the redundancy of CSF-1 in the generation of monocyte precursors,122 effectively supporting the conclusions from the op/op mouse in which there is no reduction of total circulating monocytes.28 Conversely, AFS98 has been used in several studies to address the functional role of CSF-1 signaling in pathology. For example, Murayama et al found that injection of the antibody (2 mg/day) on alternate days for 6 weeks reduced macrophage accumulation in atherosclerotic lesions in apoE-deficient mice.123 Similarly, Jose et al found that daily administration of AFS98 greatly reduced local proliferation of infiltrating macrophages in renal allografts.83 Lim et al injected similar doses for 6 weeks and observed suppression of macrophage accumulation in a model of diabetic nephropathy,124 and Segawa et al found that AFS98 administration reduced macrophage infiltration into damaged skeletal muscle.125 In the latter case, the effect was to increase fibrosis, in keeping with the antifibrotic actions of CSF-1–stimulated macrophages noted in the renal ischemia-reperfusion model discussed above. Kubota et al reported that daily AFS98 treatment (50 mg/kg) reduced macrophage numbers in an implanted osteosarcoma model and reduced vascularization, lymphangiogenesis, and tumor growth.126 Lenzo et al found that AFS98 treatment reduced the accumulation of exudate macrophages in two peritoneal and one lung inflammation model.127
Results with AFS98 contrast to the conclusions obtained using a different monoclonal anti–CSF-1R antibody, M279, which binds the receptor with significantly greater affinity.69 Prolonged treatment with this antibody selectively removed tissue macrophage populations, including those found in growing tumors, but had no protective effect in several inflammatory models, including lipopolysaccharide-induced lung inflammation, tissue injury, GVHD, or the same peritoneal model studied by Lenzo et al.127 The tumor studies were rather differently designed than those of Kubota et al126 because the tumor was allowed to establish before treatment commenced and M279 anti–CSF-1R was still able to deplete the macrophages. CSF-1–dependent macrophages may have many roles in tumor progression and metastasis, and in humans the receptor may itself be expressed by tumor cells.6,128,129 In our studies, we saw no effect of anti–CSF-1R on tumor growth, despite the macrophage depletion, but that finding does not preclude possible applications in cancer therapy.
Interestingly, the M279 antibody treatment actually led to exacerbation of GVHD, an outcome later confirmed using AFS98 by others.96 In our study using M279,69 we argue that CSF-1–dependent tissue macrophages generate peripheral tolerance and their removal permits excessive alloreactive T-cell activation. In the subsequent study,96 the authors argue that CSF-1–dependent cells are involved in the clearance of the autoreactive T cells (host vs graft), a claim connected to the apparent protective effect of pretreatment with CSF-1 in the same model discussed above.
Consistent with the lack of effect on inflammation, prolonged M279 antibody treatment of mice had no effect on the total monocyte count. Treatment ablated the more mature F4/80hiLy6Clo monocyte subpopulation, with a corresponding increase in the Ly6Chi cells suggesting a maturation block (Figure 2). As expected, based on the function of these cells as precursors of resident macrophages,64 with time, the treatment removed the large majority of tissue macrophages. The exceptions were the macrophages of the lung, brain, uterus, and ovary, and subpopulations within lymphoid tissues. The lack of impact within the brain did not reflect CSF-1 independence of microglia because these cells were depleted in nervous tissue outside the blood-brain barrier, including the retina and spinal cord. The comparative lack of effect of M279 administration on the macrophages of the uterus contrasts with a study of the role of local CSF-1 in macrophage recruitment during pregnancy,130 where treatment with AFS98 caused almost complete macrophage depletion in the uterus within 3 days. There is certainly a substantial depletion of macrophages in the uterus of the op/op mouse,131 so the lack of effect of M279 is probably the result of the local production of CSF-1 in the uterus and long half-life of the resident macrophages in the virgin females.130 In other sites, the pattern of response to M279 anti–CSF-1R was entirely consistent with the CSF-1 dependence of tissue macrophage populations based on examination of the op/op and CSF-1r (−/−) mice, the known turnover rates of tissue macrophages, and the known function of ly6Chi monocytes in inflammation.64,65,69
The acute loss of monocytes and tissue macrophages seen with AFS98 in the majority of studies suggests that this antibody, unlike M279, is either directly toxic, or it promotes clearance of monocytes/macrophages. Indeed, the effects of the antibody in the GVHD model of Hashimoto et al were mimicked by toxic liposomes.96 This finding immediately raises additional issues about interpretation and mechanism because macrophages on the surface of bone are a critical part of the stem niche, and their depletion leads to stem cell mobilization.132 Within days, AFS98 administration caused much greater depletion of splenic96 and uterine130 macrophages than we observed after prolonged treatment with M279. Among several differences between the antibodies, M279 is a rat IgG1, which is bound poorly by mouse FcRn, which regulates the half-life in the serum, whereas AFS98 is a rat IgG2A, which binds with high affinity.133 So, the doses of IgG2A used by Hashimoto et al (1-2 mg/injection) could generate sustained very high circulating concentrations of rat IgG2A.96 Second, IgG2A binds selectively to the high affinity cytophilic antibody receptor (CD64) on mouse macrophages134 and could trigger recognition by and/or aggregation with other macrophages. The effect of M279 seems more likely to represent the biology of CSF-1R signaling as opposed to less specific macrophage depletion seen with AFS98.
CSF-1R kinase inhibitors
The major difference between antibodies and kinase inhibitors is that the latter are more likely to block autocrine actions of endogenous CSF-1, which is less accessible to antibodies. Irvine et al demonstrated that a novel CSF-1R kinase inhibitor could repress autocrine signaling in mouse inflammatory macrophages, leading to reduced expression of inflammatory cytokines.45 GW2580 is probably the most selective and best characterized of the available inhibitors. There are limited pharmacologic data on the inhibitor.115,135 Treatment of mice with the drug had a small effect on thioglycollate-elicited macrophage recruitment115 ; and in a later study, despite relatively high dosing twice daily, it had limited effects in a rat arthritis model.135 Rather remarkably, there was actually a dose-related increase in blood monocyte count in normal rats after prolonged treatment.135 Priceman et al treated mice with even higher doses of GW2580 (160 mg/kg) to test the role of CSF-1R in recruitment of macrophages into growing tumors.136 Despite this increased dose, there was a small decrease in tumor macrophages and no change in the numbers of monocytes/macrophages in marrow, peripheral blood, or spleen of naive mice, by contrast to the substantial effects on tissue macrophage numbers claimed by Hashimoto et al using a significantly lower dose (1.6 mg/mouse, ∼ 60 mg/kg) only once daily, after 6 days.96
The only other CSF-1R inhibitor for which there are significant data in vivo is Ki20227. Kubota et al reported in their study of tumor angiogenesis that the inhibitor at 50 mg/kg daily subcutaneously had similar effects to AFS98 on the numbers of macrophages in tumors, and consequent angiogenesis.126 The same inhibitor was found to reduce macrophage numbers and associated pathology in models of inflammatory arthritis137 and encephalomyelitis.138 Even after prolonged treatment, the inhibitor caused only a marginal increase in circulating CSF-1 levels,138 by contrast to the massive escalation seen in CSF-1R–deficient mice.28 This implies that the inhibitor does not significantly reduce the numbers of CSF-1R–expressing tissue macrophages that normally clear the ligand from the circulation.47 Conversely, the inhibitor did greatly reduce the numbers of Ly6G-positive granulocytes which is not seen with either of the anti–CSF-1R antibodies, so there must be some concerns as to its specificity. There are several other less specific inhibitors that can block CSF-1R kinase activity but have broader specificity for other kinases. One orally available molecule, JNJ-28312141,139 has substantial activity against CSF-1R and the related Flt3. This inhibitor was found to decrease Kupffer cell numbers by approximately 40% and generated a somewhat larger increase in circulating CSF-1 (still < 2-fold) than Ki20227. Like the other molecule, Ki20227, the JNJ inhibitor reduced macrophage numbers in transplanted tumors and constrained tumor growth.139 Another molecule from the same company, dubbed fms-I, has been studied in the ureteric obstruction model of nephritis and in a glomerulonephritis model in rats.80 This inhibitor at low doses prevented local monocyte proliferation, and at higher doses drastically depleted blood monocytes and resident tissue macrophages, at least in the kidney. A molecule that inhibits CSF-1 kinase activity that is already in widespread clinical use is imatinib (STI571, Gleevec), which was originally developed as an inhibitor of ABL kinase for the treatment of chronic myeloid leukemia.140 Paniagua et al found that imatinib and GW2580 have similar efficacy in 3 different mouse models of autoimmune arthritis.141 We have tested the effect of maximally tolerated doses of imatinib using the MacGreen reporter mice. Even at doses that caused neutropenia and liver damage, we saw no evidence of the depletion of monocytes or resident tissue macrophages seen with M279 antibody (D.A.H., unpublished observations, September 2006). Dose-limiting toxicity probably occurs through inhibition of other kinases at concentrations below those required to inhibit CSF-1R kinase in vivo. Aside from these examples, none of the other patented CSF-1R inhibitors reviewed recently114 has made it into published animal trials, let alone human treatments.
In conclusion, CSF-1 as a candidate therapeutic agent is undergoing something of a renaissance in the context of tissue repair. IL-34 has not yet been tested, and we do not yet know whether it could target other receptor(s) as well as CSF-1R. A constraint on its development is that human IL-34 is not active on rodent cells,27 and it does not refold from bacterial expression. Extrapolation from animal studies of CSF-1 to humans needs to be approached with caution; a large animal model, such as the pig,142 could be a sensible intermediate. Furthermore, applications of CSF-1 must still be approached cautiously; there is the potential to make macrophage-mediated pathology considerably worse if it is applied early in the onset of disease.
The impact of the M279 anti–CSF-1R antibody in the mouse, combined with the effects of CSF-1 treatment and the phenotype of the CSF-1–deficient mouse and rat, suggests that the indispensible function of the CSF-1R signaling is to promote monocyte maturation to form the precursors of resident tissue macrophages. The available data clearly demonstrate that CSF-1R is not a good target for anti-inflammatory therapy. The high levels of CSF-1 seen in many inflammatory diseases are probably a part of a feedback damage repair response, and on that basis it could even be counterproductive to target the receptor. The exception may be where immunosuppression in certain infectious diseases and cancer is associated with very high levels of CSF-1, in which case anti–CSF-1R mAb treatments, in contrast to tyrosine kinase inhibitors, could restore effective immunity.
Anti–CSF-1R treatment could well provide a route to manipulation of macrophage numbers in tumors, where they are basically the resident tissue macrophages of a new organ. One application for anti–CSF-1R would be as an adjunct therapy to prevent regrowth after surgical or therapy-associated regression, a circumstance in which large numbers of macrophages are recruited and will otherwise contribute their trophic functions to regrowth of the tumor. The trophic role of macrophages in regrowth was first demonstrated in the op/op mouse, where transplantable tumor growth and vascularization are severely compromised.143 By analogy, CSF-1 deficiency in mice is associated with deficient hepatocyte proliferation after partial hepatectomy.144 Against that benchmark, we argue that the AFS98 antibody and the various kinase inhibitors probably elicit distinct effects through distinct mechanisms that may, or may not, involve CSF-1R signaling. In a sense, that is unimportant. As in the case of imatinib, which probably does not act solely as an Abl kinase inhibitor, the mechanism may end up being unrelated to the original hypothesis, but efficacy and therapeutic index are all that matters.
Authorship
Contribution: D.A.H. and K.P.A.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David A. Hume, Roslin Institute, University of Edinburgh, Easter Bush, Midlothian EH25 9RG, Edinburgh, United Kingdom; e-mail: david.hume@roslin.ed.ac.uk.