Abstract
Chronic myeloid leukemia (CML) is composed of 3% of pediatric leukemias, making evidence-based recommendations difficult. Imatinib has revolutionized the treatment for adult CML by eliminating allogeneic stem cell transplantation for almost all patients in chronic phase. Shown effective in pediatric CML, imatinib and successive tyrosine kinase inhibitors (TKI) have provided more therapeutic options. Because stem cell transplantation has been better tolerated in children and adolescents, the decision to treat by either TKI or transplantation is controversial. We present a recent case of a 12-month-old boy diagnosed with BCR-ABL+ CML to highlight the controversies in treatment recommendations. We review the pediatric stem cell transplantation outcomes as well as the pediatric experience with imatinib and other TKIs. Finally, we compare the side effects as well as costs associated with allogeneic stem cell transplantation versus TKI therapy. We recommend that frontline therapy for pediatric CML in chronic phase is TKI therapy without transplantation. Patients in accelerated or blast crisis or who fail to reach landmarks on TKIs either because of intolerance or resistance should pursue stem cell transplantation. Although we recommend adopting adult clinical experience to guide therapeutic decision making, the issues of infant CML, drug formulation, pharmacokinetics, and adolescent compliance merit clinical investigation.
Introduction
A 12-month-old previously healthy baby boy presented to his pediatrician with pallor and marked splenomegaly. The patient was a product of in vitro fertilization and had no siblings. (An increased incidence in early childhood leukemias for products of in vitro fertilization has been reported.1 ) The spleen was palpable down to the pelvis and 3 cm across the midline. Results of the complete blood count and differential showed white blood cell (WBC) count 335 × 103/μL with 17% neutrophils, 18% bands, 6% eosinophils, 5% basophils, 19% metamyelocytes, 8% myelocytes, 1% promyelocytes, 6% blasts, 5% monocytes, 14% lymphocytes, and 4% nucleated red blood cells, hemoglobin 6.2 g/dL, and platelets 713 × 103/μL. Serum electrolytes, blood urea nitrogen, and creatinine were within normal limits, but the serum lactate dehydrogenase was markedly elevated at 1465 U/mL. Bone marrow aspirate revealed 12% myeloid blasts. Cytogenetics and FISH showed t(9;22)(q34;q11). The cerebrospinal fluid was normal.
The patient received hydroxyurea 50 mg/kg per day on day 1, and increased to 75 mg/kg per day on day 3. Imatinib 340 mg/m2 per day was begun on day 4 after t(9;22) was confirmed. Based on bone marrow blast count and confirmation of Bcr-Abl, the infant was diagnosed as chronic myeloid leukemia (CML) in accelerated phase. The patient's WBC count dropped to 176 × 103/μL at day 7 and 33 × 103/μL at day 14, at which point hydroxyurea was discontinued. By day 21, the patient's WBC count decreased to the normal range (9 × 103/μL). The patient did extremely well with no organ dysfunction or clinical events. The patient was continued on imatinib 340 mg/m2 per day, which was well tolerated.
The patient obtained a complete hematologic response (CHR) within 1 month of diagnosis. At 6 months, he continued to have persistent cytogenetic evidence of t(9;22) and rising Bcr-Abl transcript levels. Bcr-Abl kinase domain mutational analysis was performed and revealed no mutations. Imatinib was discontinued, and the patient started therapy with dasatinib 100 mg/m2 per day. At 18 months from diagnosis, the family agreed to proceed to matched unrelated donor stem cell transplantation (SCT) and requested reduced intensity conditioning. The patient underwent treatment with fludarabine, busulfan, and anti–thymocyte globulin and received peripheral blood stem cells from a 10 of 10 HLA-matched donor. GVHD prophylaxis consisted of cyclosporine and mycophenolate mofetil. Unfortunately, the patient developed grade 4 acute GVHD of the gut and eventually died from its complications 4 months after transplantation.
This case illustrates the complexity of managing a child with CML, with the malignant clone's biologic features identical to the adult disease but with markedly different host properties. Do infants with CML behave similarly to older pediatric and adult patients? How valid in pediatrics are adult-derived response landmarks and time to reach them? Does formulation of oral TKI affect pharmacokinetics and outcome? How does one determine the dose for infants and children? Are there age-specific differences in TKI pharmacokinetics? When does one consider SCT in a population of patients who are supposed to tolerate the procedure better and with less GVHD? How much is parental/individual compliance a problem in pediatrics? Are there very long-term side effects of TKI?
CML is composed of 3% of newly diagnosed pediatric leukemias, with an annual incidence of approximately 1 per million children and adolescents younger than 20 years.2 CML is even rarer in children younger than age 4, with only 2 published case reports of children in this age group.3,4 There appears to be no ethnic or genetic predisposition. Although ionizing radiation is a risk factor for development of the disease, this and other environmental exposures have not been demonstrated to be causal in children.
Children are known to present with a higher median WBC count, although otherwise present nearly identically to adults.4,5 CML most commonly presents with fatigue, asthenia, and splenomegaly.4-6 Symptoms and signs of bone marrow infiltration or hyperleukocytosis may also be seen in more advanced cases. The natural history of pediatric CML progresses through 3 phases, similar to adult CML. Chronic phase (CML-CP) results in expansion of hematopoiesis and is defined by less than 10% bone marrow blasts. Children with CML-CP typically present with leukocytosis, anemia, and thrombocytosis.4 Children tend to present with higher WBC counts than adults, with a median WBC count of 250 000 × 103/μL.4,5 According to the WHO classification, accelerated phase (CML-AP) is defined by the following: 10% to 19% bone marrow blasts, peripheral blood basophils more than 20%, persistent thrombocytopenia (< 100 × 109/L or thrombocytosis (> 1000 × 109/L), increasing spleen size or increasing WBC count unresponsive to therapy, or cytogenetic clonal evolution.7 CML-AP occurs less commonly in pediatrics. Blast crisis (CML-BC) presents as overt acute leukemia and is defined by more than or equal to 20% bone marrow blasts or extramedullary blast proliferation.7 See Table 1 for diagnostic criteria for CML by stage. Approximately 95% of children will present in chronic phase, with the remainder presenting in advanced stages.5 Adult presentation by phase is very similar, with 93% of adults presenting in chronic phase.6
Criteria for chronic, accelerated, and blast phases of CML7
| CML-CP . | CML-AP . | CML-BC . |
|---|---|---|
| Must meet all of the following criteria: | Must meet 1 or more of the following criteria: | Must meet 1 or more of the following criteria: |
| Documentation of t(9;22) or the Bcr-Abl fusion gene | Blasts 10%-19% of peripheral blood white cells or bone marrow cells | Blasts ≥ 20% of peripheral blood white cells or bone marrow cells |
| Bone marrow blasts < 10% | Peripheral blood basophils at least 20% | Extramedullary blast proliferation |
| Does not meet any criteria for accelerated phase or blast crisis | Persistent thrombocytopenia (100 × 109/L) unrelated to therapy, or persistent thrombocytosis (1000 × 109/L) unresponsive to therapy | Large foci or clusters of blasts in bone marrow biopsy |
| Increasing spleen size and increasing WBC count unresponsive to therapy | ||
| Cytogenetic evidence of clonal evolution (ie, the appearance of an additional genetic abnormality that was not present in the initial specimen at the time of diagnosis of chronic phase CML) | ||
| Megakaryocytic proliferation in sizable sheets and clusters, associated with marked reticulin or collagen fibrosis, and/or severe granulocytic dysplasia, should be considered as suggestive of CML-AP; these findings have not yet been analyzed in large clinical studies; however, so it is not clear whether they are independent criteria for accelerated phase; they often occur simultaneously with one or more of the other features listed |
| CML-CP . | CML-AP . | CML-BC . |
|---|---|---|
| Must meet all of the following criteria: | Must meet 1 or more of the following criteria: | Must meet 1 or more of the following criteria: |
| Documentation of t(9;22) or the Bcr-Abl fusion gene | Blasts 10%-19% of peripheral blood white cells or bone marrow cells | Blasts ≥ 20% of peripheral blood white cells or bone marrow cells |
| Bone marrow blasts < 10% | Peripheral blood basophils at least 20% | Extramedullary blast proliferation |
| Does not meet any criteria for accelerated phase or blast crisis | Persistent thrombocytopenia (100 × 109/L) unrelated to therapy, or persistent thrombocytosis (1000 × 109/L) unresponsive to therapy | Large foci or clusters of blasts in bone marrow biopsy |
| Increasing spleen size and increasing WBC count unresponsive to therapy | ||
| Cytogenetic evidence of clonal evolution (ie, the appearance of an additional genetic abnormality that was not present in the initial specimen at the time of diagnosis of chronic phase CML) | ||
| Megakaryocytic proliferation in sizable sheets and clusters, associated with marked reticulin or collagen fibrosis, and/or severe granulocytic dysplasia, should be considered as suggestive of CML-AP; these findings have not yet been analyzed in large clinical studies; however, so it is not clear whether they are independent criteria for accelerated phase; they often occur simultaneously with one or more of the other features listed |
Like adults, most children will present in chronic phase.
Is the biology the same in adults and pediatrics?
Pediatric CML shares with adult CML the same molecular feature: the balanced translocation t(9;22)(q34;q11) that results in the fusion gene BCR-ABL.8 When pediatric Ph+ leukemias were studied genetically, the t(9;22) breakpoints occurred in the known breakpoint cluster regions in the BCR gene on chromosome 22.9 BCR-ABL encodes a constitutively active tyrosine kinase, either as a 210 kDa in CML or 190 kDa in B-cell lineage acute lymphoblastic leukemia. The multistep pathogenesis of CML leukemogenesis was thought to require years to complete. However, given our case report and other reports of infant CML, the transformative events may occur over a short time period.
No biologic differences in pathway activation have been noted between patient populations. However, host biologic differences affecting drug clearance or morbidity associated with allogeneic transplantation do exist. Little is known about pharmacokinetics of TKI in children and infants. In addition, variability in pharmacy formulation may affect absorption. Other host factors affect SCT outcome, which tend to be improved in children. Better outcomes for pediatric transplant patients have been attributed to the lack of chronic diseases or organ dysfunction associated with age and decreased incidence of GVHD. SCT outcomes are also similar between children and adults, although outcomes may be slightly better in children. These host differences are discussed in “SCT in pediatric CML.”
SCT in pediatric CML
Before the advent of imatinib, allogeneic SCT was the standard of care for all children with CML. The use of donor lymphocyte infusions to treat recurrences after transplantation has been effective therapy for many patients and demonstrates that a graft-versus-leukemia effect is an important mechanism by which transplant cures for CML.10
Transplant outcomes, including acute and chronic morbidity and costs, should be used as a baseline with which to judge the newer small-molecule-based therapies. Direct comparison of the 2 treatment modalities is difficult because of confounding factors, such as greater disease burden before TKI use, greater use of reduced intensity conditioning (RIC), and improved supportive care (eg, fungal and viral prophylaxis).
A limited number of retrospective reports for SCT exist before the imatinib's use in pediatric CML.11-14 For children with CML-CP who received matched sibling donor transplants from 1982 to 2004, 3- to 5-year event-free survival (EFS) ranged from 61% to 63%, whereas overall survival (OS) ranged from 66% to 87%.11,12,14 For matched unrelated donors, outcomes were slightly worse, with EFS 27% to 55% and OS 45% to 65%.11-14 Morbidity was significant, with grade 2 to 4 acute GVHD seen in 28% to 37% of matched-sibling donor transplants and 52% to 80% of matched-unrelated donor transplants; chronic GVHD (cGVHD) was also common (38%-44%). cGVHD can be a devastating and long-term complication of SCT. In a series where long-term follow-up of pediatric patients with cGVHD is available, 15% of patients have ongoing cGVHD many years after transplantation and cGVHD can contribute to late deaths in 12% of patients.15 In a very recent series of pediatric patients transplanted for leukemia or myelodysplastic syndrome, cGVHD is associated with significant mortality: 5-year nonrelapse mortality was 24% from the time of cGVHD diagnosis.16 Among adult survivors of allogeneic SCT, both acute and cGVHD have been shown to have a negative impact on quality of life in several studies.17
Adults and children who are transplanted in accelerated phase, blast crisis, or second chronic phase have worse outcomes.12 In addition, time to transplantation has also been associated with worse outcomes.18,19 However, these data are before the introduction of TKIs and are probably now obsolete. TKIs have altered the landscape of therapy, with significant lengthening of median time to transplantation.
In both mice and humans, donor lymphocyte infusions are effective in relapsed CML, lending evidence toward graft-versus-leukemia activity.20,21 Because of observed graft-versus-leukemia effect in CML, RIC is appealing. Although RIC has decreased transplant-related mortality and may be associated with fewer long-term side effects, GVHD remains a significant and common problem for these patients. Several small series of adult patients who have undergone RIC produced EFS 70% to 100%.22-24 In adults with CML who underwent RIC transplantation, advanced disease at the time of transplantation was the major factor associated with reduced OS and progression-free survival, suggesting that RIC transplants may have a limited role in patients with advanced disease. Patients with significant gross residual disease probably have too great a tumor burden to benefit from the graft-versus-leukemia effect.25,26 To date, there have not been published data confirming these results in children. A reduced intensity regimen of busulfan, fludarabine, and rabbit anti–thymocyte globulin was used in 2 children with CML with success in one recent report.27 RIC transplantation remains appealing for pediatric CML, but additional clinical experience is required before RIC can be accepted as the standard of care.
Whether introduction of imatinib or TKI affects SCT results remains uncertain. The Center for International Blood and Marrow Transplant Research performed a retrospective analysis, comparing outcomes of patients who received TKIs before transplantation with patients who did not. Patients who received imatinib before transplantation had a significantly lower risk of death compared with patients who did not receive imatinib.28 TKI leads to lower disease burden at time of transplantation, which might decrease the likelihood of relapse after transplantation. The role of TKI therapy after transplantation is unclear. Imatinib has been used to treat cGVHD, suggesting that its use may prevent relapse and treat cGVHD.29 In published reports of prophylactic TKI therapy after transplantation, the time to initiate TKI therapy has been highly variable.30-32 However, TKIs have been well tolerated after transplantation without significant toxicities, establishing the feasibility of this approach.30-32 Many pediatric and adult centers use TKIs after transplantation for up to 1 year.
TKI in CML
The first-generation TKI imatinib received accelerated approval for CML in adults in 2001 and in pediatrics in 2003. The second-generation TKIs dasatinib and nilotinib won FDA approval in 2006 and 2007, respectively, for adult patients with Ph+ leukemia who were intolerant or resistant to imatinib and have recently shown superiority to imatinib for front-line therapy.33,34 The small-molecule inhibitor binds to the inactive conformation of BCR-ABL, whereas second-generation TKIs bind to both the active and inactive conformations. The second-generation TKIs also demonstrate a 2-log greater sensitivity than imatinib and are also active in resistance-associated mutations in BCR-ABL. The T315I mutation, resulting from the substitution of threonine for isoleucine at amino acid residue 315, results in a physical, steric hindrance of imatinib binding to the ATP-binding pocket of the BCR-ABL kinase.35 This mutation occurs in approximately 12% of adult patients with BCR-ABL leukemias and is more likely to be identified in advanced stage disease.36 Unfortunately, neither dasatinib nor nilotinib is active against T315I.
Effectiveness of TKI therapy is determined by the achievement of landmark responses: hematologic, cytogenetic, and molecular (Table 2). CHR is defined by the following values: WBCs less than 10 × 109/L; platelets less than 450 × 109/L; and myelocytes and metamyelocytes less than 5% in peripheral blood, no blasts, and promyelocyte in peripheral blood, and basophils less than 20% with no extramedullary involvement. A major cytogenetic response (MCyR) includes both complete and partial response. Complete cytogenetic response (CCyR) is defined when there are 0% Ph+ metaphases. A minor cytogenetic response is defined when there are between 35% and 67% Ph+ metaphases, and partial cytogenetic response (PCyR) is defined when there are 1% to 35% Ph+ metaphases in marrow culture. Quantitative PCR has emerged as the most sensitive marker of detection of Bcr-Abl and is able to detect 1 in 100 000 cells (10−6), although this varies by laboratories. Complete molecular response is defined as undetectable transcript or a 3-log reduction in transcript from diagnosis by quantitative PCR. A major molecular response (MMR) is defined by a 3-log reduction of the Bcr-Abl transcript.
| Hematologic response: CBC . | Cytogenetic response: Ph+ metaphases . | Molecular response: Bcr-Abl transcripts . |
|---|---|---|
| Complete: WBC < 10 × 103/L, platelets < 450 × 109/L, and differential with no immature granulocytes and < 5% basophils | Complete: 0% | Complete: transcripts not detectable, or > 3-log reduction in transcript from diagnosis |
| Partial: 1%-35% | Major: < 0.1% | |
| Minor: 36%-65% | ||
| Minimal: 66%-95% |
| Hematologic response: CBC . | Cytogenetic response: Ph+ metaphases . | Molecular response: Bcr-Abl transcripts . |
|---|---|---|
| Complete: WBC < 10 × 103/L, platelets < 450 × 109/L, and differential with no immature granulocytes and < 5% basophils | Complete: 0% | Complete: transcripts not detectable, or > 3-log reduction in transcript from diagnosis |
| Partial: 1%-35% | Major: < 0.1% | |
| Minor: 36%-65% | ||
| Minimal: 66%-95% |
TKI success in adults
Imatinib has revolutionized therapy for adult CML. The International Randomized Study of Interferon and STI571 trial included 553 patients with CML demonstrated CHR in 95% and OS of more than or equal to 95% at 1.5 years for patients in chronic phase.37 This excellent response continues at now more than 6 years of imatinib therapy. Best responses included CHR in 97% and CCyR in 82%.38 EFS and OS at 6 years were 83% and 88%, respectively.38 In addition, while considering only CML-related deaths, OS reached 95%.38 Data at 8 years on imatinib have also been presented in abstract form and show continued good response, with OS of 92% when considering only CML-related deaths.39 Annual rates of progression in years 4 to 8 range between 0% and 0.9%.39 These results are incredibly impressive and underscore the importance of upfront imatinib for patients with CML. For adults, optimal responses include obtaining CHR at 3 months, PCyR at 6 months, CCyR at 12 months, and MMR at 18 months.40 The European LeukemiaNet panel has developed a consensus for failure and suboptimal responses (Table 3).40,41
Time landmarks and response criteria to TKI
| Time, mo . | Failure . | Suboptimal response . | Warnings . |
|---|---|---|---|
| Diagnosis | NA | NA | High risk; del(9q-); additional cytogenetic abnormalities in Ph+ cells |
| 3 | No HR; stable disease or disease progression | Less than CHR | NA |
| 6 | Less than CHR; no cytogenetic response: Ph+ > 95% | Less than PCyR; Ph+ > 35% | NA |
| 12 | Less than PCyR; Ph+ > 35% | Less than CCyR | Less than MMR |
| 18 | Less than CCyR | Less than MMR | NA |
| Anytime | Loss of CHR; loss of CCyR; mutation (ex. T315I) | Additional cytogenetic abnormalities in Ph+ cells; loss of MMR; mutation | Any rise in transcript level; additional cytogenetic abnormalities in Ph− cells |
| Time, mo . | Failure . | Suboptimal response . | Warnings . |
|---|---|---|---|
| Diagnosis | NA | NA | High risk; del(9q-); additional cytogenetic abnormalities in Ph+ cells |
| 3 | No HR; stable disease or disease progression | Less than CHR | NA |
| 6 | Less than CHR; no cytogenetic response: Ph+ > 95% | Less than PCyR; Ph+ > 35% | NA |
| 12 | Less than PCyR; Ph+ > 35% | Less than CCyR | Less than MMR |
| 18 | Less than CCyR | Less than MMR | NA |
| Anytime | Loss of CHR; loss of CCyR; mutation (ex. T315I) | Additional cytogenetic abnormalities in Ph+ cells; loss of MMR; mutation | Any rise in transcript level; additional cytogenetic abnormalities in Ph− cells |
These landmarks were established based on imatinib trials. For the second-generation TKI, these will probably be revised in the next year based on adult responses. Whether time to reach landmarks with the more potent second-generation TKI results in comparable clinical outcomes is not currently known. The most important landmark and predictor for success is complete cytogenetic response.
NA indicates not applicable.
For adults presenting in accelerated phase or blast crisis, imatinib demonstrates good activity, although response rates are significantly decreased from the 95% CHR rate and more than 95% OS seen in adults in chronic phase at 1.5 years.37 For those treated in accelerated phase, the CHR rate was only 34% and the corresponding OS was 74% at 1 year.42 For those treated in myeloid blast crisis, CHR was seen in 8% and median time to progression was 10 months.42 Dasatinib has been used in adults with advanced CML, many of whom had imatinib-resistant disease or who were intolerant to imatinib. In a series of 174 patients reported by Apperley et al,43 CCyR was achieved in only 31% of patients with median follow-up of 14 months.
Quantitative PCR has allowed detection of minimal residual disease. Imatinib has performed well in reducing disease burden, with 39% of early chronic phase adults with CML obtaining a more than 3-log reduction in BCR-ABL transcript 12 months from diagnosis.44 Approximately 15% to 31% of patients will have evidence of cytogenetic resistance, as defined by suboptimal cytogenetic response at 12 months.45 Resistance to imatinib may occur through either primary or secondary mechanisms. Primary resistance may be the result of pharmacokinetic variations, including abnormalities in transport or drug efflux. More often, patients develop secondary or acquired resistance through mutations of Bcr-Abl or recruitment of salvage pathways. The T315I mutation is the most feared mutation, as it is resistant to all FDA-approved TKIs. However, several agents that can abrogate kinase activity associated with T315I, such as ponatinib, are in clinical trials.46
Some have advocated an increase in the imatinib dose for patients with suboptimal response. Jabbour et al recently reported 84 patients with imatinib failure who received dose escalation, typically from 400 to 800 mg daily (a small number of patients were escalated from 300 to 600 mg).47 Forty percent of patients were able to obtain a complete cytogenetic response. However, responses were not durable, with 2-year EFS of 57% and 7-year EFS of only approximately 10%.47
Recent large clinical trials have shown impressive response rates for the second-generation TKIs. First-line dasatinib therapy had improved response rates for CML-CP compared with imatinib.33 After 1 year of therapy, rates of CCyR were 77% versus 66% for dasatinib and imatinib therapy, respectively.33 MMR rates were also higher with dasatinib, 46% versus 28%. The side effect profile of dasatinib was similar to imatinib. A similar multinational study showed that upfront nilotinib also had improved response rates compared with imatinib.34 Again, after 1 year of therapy, CCyR was achieved in 80% of patients receiving nilotinib versus 65% with imatinib therapy.34 Similarly, MMR rates were higher with nilotinib, 44% versus 22%. Again, the side effect profiles were quite similar to imatinib. However, a mortality difference has not been observed between the first- and second-generation TKIs. These 2 large studies have raised the issue that one may consider a second-generation TKI for front-line therapy for CML. The National Comprehensive Cancer Network guidelines were recently updated in 2011 to include dasatinib and nilotinib as well as imatinib as first-line options for CML in adults.
TKI success in pediatrics
Limited data on the use of TKI in pediatric CML are available. One group estimated that the efficacy of imatinib has been evaluated in less than 200 children to date (as of 2010).48 The landmark phase 1 trial from the Children's Oncology Group evaluated 31 children with leukemia who received imatinib at doses ranging from 260 to 570 mg/m2.49 Imatinib was extremely well tolerated, and a maximum tolerated dosage could not be identified. Adult doses of 400 and 600 mg equated to similar exposures in children of 260 and 340 mg/m2, respectively.49 Thus, the most commonly recommended starting dose for children is in the range of 340 mg/m2. Although this phase 1 trial was not designed for efficacy, 10 of 12 children achieved a CCyR.49
The most impressive prospective study comes from the French National phase 4 trial, which enrolled 44 children with CML-CP and treated them with imatinib 260 mg/m2.50 CHR was achieved in 98% of children.50 Rates of CCyR and MMR were 61% and 31%, respectively, at 1 year.50 Overall, at a median follow-up of 31 months, progression-free survival was 98%.50 Approximately 30% of patients had discontinued imatinib because of unsatisfactory response, as some patients were taken to SCT and some patients may have proceeded to second-generation TKIs.
A phase 2 trial of imatinib given at 260 to 340 mg/m2 (rounded to avoid splitting 100-mg capsules) was conducted in Europe consisting of 30 high-risk children with CML in late chronic (as defined by resistant or intolerant to interferon), advanced stages, or for relapse after SCT.51 Responses were excellent with CHR achieved in 80% and CCyR in 60% of late chronic phase children.51 For patients in advanced stages, CHR was achieved in 75% and CCyR in 29%.51 Estimated 1-year OS was 95% and 75% for patients in late chronic phase and advanced stages, respectively.
Kolb et al reported 5 consecutive children with Ph+ leukemia: 4 with CML and one with mixed lineage leukemia.52 These 5 children were started on imatinib, and each achieved CHR, CCyR, and CMR at a median of less than 30, 285, and 287 days, respectively.52 Imatinib was again well tolerated, and there were no dose-limiting side effects identified. Imatinib has also been given for solid tumors in children without adverse events. A phase 2 Children's Oncology Group study documented no objective responses to relapsed or refractory solid tumors.53
Based on the impressive adult data with second-generation TKIs, there is significant interest in dasatinib as an agent for children with BCR-ABL+ leukemias. A recent phase 1 trial from the Children's Oncology Group evaluated escalating doses of dasatinib in 39 children with refractory solid tumors or leukemias.54 All 8 evaluable patients with CML had a response to dasatinib, including 3 complete cytogenetic responses and 3 partial cytogenetic responses.54 Currently, there is an active Children's Oncology Group trial (AALL0622) for children with Ph+ acute lymphoblastic leukemia (ALL). In addition, Bristol-Myers Squibb currently has an open phase 2 dasatinib trial (NCT00777036) for newly diagnosed pediatric CML-CP.
Information about the use of nilotinib in children is limited. Reporting on 16 pediatric patients in its compassionate use program, Wayne et al dosed patients more than 40 kg at 400 mg twice a day and less than 40 mg at 300 mg twice a day.55 Half of the patients had Ph+ ALL and 5 had either CML-AP or CML-BC. Although nilotinib had a safety profile similar to adults, it provided little benefit for those who failed imatinib and dasatinib.55
TKI pharmacokinetics
Compared with adult patients, information about TKI pharmacokinetics in children is sparse. Imatinib is completely absorbed after oral administration and is 95% bound to plasma proteins.56 Imatinib is broken down into its major metabolite CGP-74588. Both molecules have similar pharmacokinetic properties and are metabolized in the liver by CYP3A4 and CYP3A5 enzymes.57 A comprehensive list of drugs that interfere with the cytochrome P450 system may be found at http://medicine.iupui.edu/clinpharm/ddis. Some of the more common drugs that may increase imatinib levels include: ketoconazole, itraconazole, erythromycin, and clarithromycin. Drugs that may decrease plasma imatinib levels are: carbamazepine, phenobarbital, phenytoin, rifampin, and dexamethasone. The 15-hour half-life of imatinib in children appears to be similar, although slightly shorter, than that of the 17-hour half-life seen in adults.58 Steady-state plasma concentrations of imatinib are reached within 7 days.49 As identified in the phase 1 Children's Oncology Group imatinib study, steady-state plasma concentrations in children receiving 260 to 340 mg/m2 per day correspond to adult doses of 400 to 600 mg.49 (See Table 4 for relevant starting doses of imatinib, dasatinib, and nilotinib in children and adolescents.) Liquid formulations may be prepared and administered freshly (Table 5). However, there is significant interpatient variability in plasma concentration and other pharmacokinetic parameters.49,57,59 Similarly, dasatinib showed significant interpatient variability in pharmacokinetic properties, including plasma concentration, half-life, and clearance.54 A pharmacokinetic study of nilotinib in pediatric patients with Ph+ CML or ALL (NCT01077544) began enrollment in December 2010.
Starting doses of TKIs
| TKI . | Dose, mg/m2 . | Frequency . | Dosage strength, mg . |
|---|---|---|---|
| Imatinib | 340 | Once daily | 100, 400 |
| Dasatinib | 60-80 | Once daily | 20, 50, 70, 80, 100, 140 |
| Nilotinib* | 170-230 | Twice daily | 150, 200 |
| TKI . | Dose, mg/m2 . | Frequency . | Dosage strength, mg . |
|---|---|---|---|
| Imatinib | 340 | Once daily | 100, 400 |
| Dasatinib | 60-80 | Once daily | 20, 50, 70, 80, 100, 140 |
| Nilotinib* | 170-230 | Twice daily | 150, 200 |
Pediatric dosing has not been established but is based on 300 mg or 400 mg twice daily if less than or greater than 40 kg, respectively.
Instructions to prepare liquid suspension of TKIs
| TKI . | Instructions . |
|---|---|
| Imatinib | Tablets may be dispersed in water or apple juice using 50 mL for 100-mg tablet or 200 mL for 400-mg tablet. The contents must be stirred until dissolved and used immediately. For children < 3 y old, it is recommended that at least 120 mL of water or food be taken to avoid esophageal irritation. |
| Dasatinib | Tablets can be allowed to dissolve over 20 minutes at room temperature in 30 mL of lemonade, preservative-free apple juice, or preservative-free orange juice. After ingestion, rinse the residue off glass with 15 mL of the juice and administer. |
| Nilotinib | Capsules may be dispersed in 5 mL of applesauce and ingested immediately on an empty stomach, and abstain from eating for at least 1 hour. |
| TKI . | Instructions . |
|---|---|
| Imatinib | Tablets may be dispersed in water or apple juice using 50 mL for 100-mg tablet or 200 mL for 400-mg tablet. The contents must be stirred until dissolved and used immediately. For children < 3 y old, it is recommended that at least 120 mL of water or food be taken to avoid esophageal irritation. |
| Dasatinib | Tablets can be allowed to dissolve over 20 minutes at room temperature in 30 mL of lemonade, preservative-free apple juice, or preservative-free orange juice. After ingestion, rinse the residue off glass with 15 mL of the juice and administer. |
| Nilotinib | Capsules may be dispersed in 5 mL of applesauce and ingested immediately on an empty stomach, and abstain from eating for at least 1 hour. |
There are, however, very little pharmacokinetic data for TKIs available from very young children younger than 4 years. Based on pharmacokinetics of other drugs, it is possible that infants and toddlers have increased metabolism and lower steady-state plasma concentrations of TKIs than their older counterparts. Clearly, further investigations are warranted in this age group.
TKI potential adverse events important in the pediatric population
Imatinib is generally well tolerated, and it certainly has far fewer side effects than does conventional chemotherapy. Many patients may have hematologic toxicity as evidenced by cytopenias. In adults with CML in CP, 13% of patients had grade 3 or 4 neutropenia and 7% had grade 3 or 4 thrombocytopenia.60 In children, 27% of patients had grade 3 or 4 neutropenia, 5% had grade 3 or 4 thrombocytopenia, and 2.5% had grade 3 or 4 anemia.50 The cytopenias tend to be very manageable by transiently stopping imatinib. Nonhematologic toxicities include gastrointestinal symptoms, such as nausea, vomiting, and diarrhea. Additional toxicities include rash, edema, elevated liver function tests, myalgia, and bone pain. For fluid retention, salt intake should be restricted and diuretics may be used. Mild skin rashes may be treated with topical steroids.
Cardiac toxicity, including the prolonged QT syndrome, from imatinib or second TKI can occur. Kerkela et al reported a series of 10 adults who developed congestive heart failure while on imatinib.61 There remains significant controversy regarding cardiac toxicity with imatinib, and many authors conclude that there may be no increased risk of heart dysfunction.40,48 To date, no reports of cardiac dysfunction in children have surfaced. Although it is unknown whether long-term administration of TKI affects heart function, one group observed left ventricular abnormalities in mice treated with imatinib because of c-ABL inhibition.61 Dasatinib is associated with the development of pleural effusions, as observed in 2 children in the phase 1 Children's Oncology Group study.54 Nilotinib carries a boxed warning regarding QT prolongation and sudden death and that it should not be used when hypokalemia or hypomagnesemia is present.
Growth retardation is also a concerning side effect for the pediatric population, especially for prepubertal children. Multiple case reports have demonstrated growth retardation in children on imatinib.62,63 Larger reports from Europe have confirmed growth delay. Shima et al reviewed the growth of 48 children receiving imatinib therapy and found 73% of patients had a decrease in height with median SD height decrease of 0.61 at a follow-up of 34 months.64 The negative impact of height was predominantly on prepubertal children.64 Imatinib has been shown to cause hypocalcemia and hypophosphatemia and probably has adverse effects on bone health.65 Careful monitoring of longitudinal height and bone laboratory tests is recommended.48 Imatinib may also be a teratogen, and care should be taken to avoid pregnancy while on this medication.66 See Table 6 for a summary of important TKI side effects and their management.
Major TKI side effects
| TKI side effects . | Recommended intervention . |
|---|---|
| Hematologic toxicity: neutropenia, anemia, or thrombocytopenia | Hold TKI until count recovery for up to 2 wks; G-CSF may be administered to treat neutropenia; restart at full dose if cytopenia persists < 2 wks; reduce dose by 20% if longer than 2 wks |
| Rash | Observation; consider topical steroids |
| Elevated liver function tests | Observation |
| Muscle cramps | Supportive care, consider electrolyte repletion |
| Nausea, vomiting | Supportive care, consider ondansetron |
| Headache | Supportive care |
| Edema | Restrict salt intake, consider diuretics |
| Cardiac toxicity | Consider ECG, echocardiogram, electrolytes if there is clinical concern |
| Imatinib: possible, not proven | |
| Dasatinib: possible, not proven | |
| Nilotinib: QT prolongation and sudden death have been reported | Do not use nilotinib with history of cardiac or electrolyte problems |
| Effusions | Hold TKI; if multiple sites of edema, give diuretics; and if severe, thoracentesis and brief course of steroids |
| Dasatinib: pleural effusions | |
| Decreased height/growth retardation | Closely monitor height |
| Poor bone health | Closely monitor calcium and phosphorus; replete as necessary |
| Teratogen | Avoid TKIs during pregnancy |
| TKI side effects . | Recommended intervention . |
|---|---|
| Hematologic toxicity: neutropenia, anemia, or thrombocytopenia | Hold TKI until count recovery for up to 2 wks; G-CSF may be administered to treat neutropenia; restart at full dose if cytopenia persists < 2 wks; reduce dose by 20% if longer than 2 wks |
| Rash | Observation; consider topical steroids |
| Elevated liver function tests | Observation |
| Muscle cramps | Supportive care, consider electrolyte repletion |
| Nausea, vomiting | Supportive care, consider ondansetron |
| Headache | Supportive care |
| Edema | Restrict salt intake, consider diuretics |
| Cardiac toxicity | Consider ECG, echocardiogram, electrolytes if there is clinical concern |
| Imatinib: possible, not proven | |
| Dasatinib: possible, not proven | |
| Nilotinib: QT prolongation and sudden death have been reported | Do not use nilotinib with history of cardiac or electrolyte problems |
| Effusions | Hold TKI; if multiple sites of edema, give diuretics; and if severe, thoracentesis and brief course of steroids |
| Dasatinib: pleural effusions | |
| Decreased height/growth retardation | Closely monitor height |
| Poor bone health | Closely monitor calcium and phosphorus; replete as necessary |
| Teratogen | Avoid TKIs during pregnancy |
Taken together, there are side effects from TKIs, yet they tend to be manageable. The 2 most concerning and long-term effects are cardiac dysfunction and growth delay. These toxicities must be evaluated further. Evaluation of the risks and benefits of TKIs must also be weighed against the alternative options, including allogeneic SCT.
Cost-benefit analysis
Based on direct medical costs calculated for a 2-year period, imatinib offered a cost-effective advantage compared with matched unrelated donor allogeneic bone marrow transplant.67 The cost of a one-year supply of imatinib (400 mg daily) is $64 800. The annual costs of dasatinib and nilotinib at their recommended dosage are greater ($72 000-$84 000). Total costs of allogeneic SCT vary significantly depending on the mix of inpatient and outpatient stays and whether it is a matched sibling donor or matched unrelated transplant. For pediatric leukemia patients at standard risk, the average one-year costs for an allogeneic peripheral blood SCT were $512 294 and $352 885 for allogeneic bone marrow transplant.68 After reviewing mathematical models of cost-effectiveness and quality-adjust life-years, the National Institute for Health and Clinical Excellence disapproved financial reimbursement for dasatinib or nilotinib through the British National Health Service. Their consideration rested on the lack of evidence-based stopping rule for either TKI and cost comparison of either second-generation TKI with high-dose imatinib.69 However, economic analyses may not include all costs associated with treatment modality. Difficult to model, these include the costs of SCT complications, such as the many manifestations of cGVHD, costs of inpatient and outpatient treatment associated with SCT complications, financial losses in wages or income incurred by parents because of work absences, and travel to and from the medical or SCT center. In addition, the costs for prolonged administration of TKI are difficult to estimate because of possible stopping rules or decreased costs as drugs become generic. (Imatinib will be coming off of patent protection in January 2015, and dasatinib in June 2020.) Cost-benefit analyses must also include the effects on the pediatric patient and his/her future earnings. Thus, in addition to their clinical efficacy, the TKIs may provide an economic advantage over transplantation.
CML: the importance of cure
The issue of long-term control versus cure is important when discussing therapy for pediatric CML. SCT remains the only proven cure for CML. Many pediatric providers may be uncomfortable with a therapy that is not curative. However, the Stop Imatinib trial is ongoing in adults, with the aim of determining the feasibility of discontinuing imatinib for select adults with CML.70 At the interim analysis, 100 patients with CML in CMR had been enrolled. At a follow-up of 12 months, 41% of patients had remained in CMR.70 Patients who relapsed responded to reintroduction to imatinib without adverse events,70 although with limited follow-up, this important trial raises the possibility of cure for patients with TKIs alone. Clearly, further follow-up and additional data are required before this may be considered for children.
Recommendations
Based on the similar biology and behavior of p210 Bcr-Abl CML in adults and pediatrics and the published findings of adult trials, we recommend the following guidelines for pediatric CML. We propose using similar TKI landmarks in children to evaluate TKI response, as adult groups have done successfully. Therapeutic considerations may be modified based on the tolerance and responsiveness of the pediatric age group to TKI or transplantation.
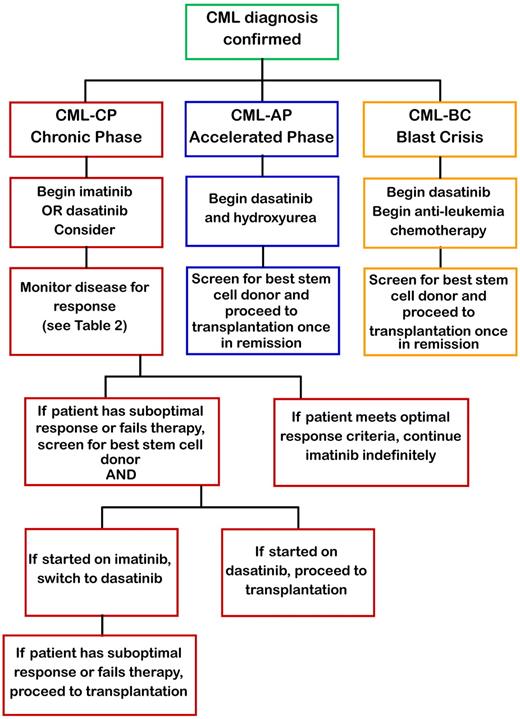
Because there have been no randomized controlled trials comparing transplant and imatinib in pediatrics, the decision on how to treat has been individualized. Because of the small number of pediatric patients with CML and recent advances in SCT conditioning regimens and availability of newer more potent TKI, recommendations on treating pediatric CML perforce rest on adult clinical data. Developed almost 30 years ago, the Sokal score has been calculated to prognosticate by defining patients as low, intermediate, and high risk. Although the Sokal score has found some utility in the TKI era, its reliance on age and spleen size precludes its application to pediatrics. Treatment guidelines may be modified from the adult experience because of the host factors, such as pharmacokinetics, compliance during adolescence, effects of TKI on growth and puberty, and theoretically several decades' longer period of drug treatment. Although data are emerging to show that second-generation TKIs provide a more rapid response and greater 3-year EFS compared with standard-dose or high-dose imatinib, we practicing pediatric oncologists and stem cell transplanters recommend the following (Figure 1):
Chronic phase CML
For children presenting with CML in chronic phase, begin treatment with hydroxyurea 25 to 50 mg/m2 per day and start imatinib at 340 mg/m2 per day once daily on confirmation of Bcr-Abl by FISH and continue this medication indefinitely. If covered by third party payer, treatment with dasatinib 60 mg/m2 per day may also be considered.
Monitor disease status through quantitative PCR every 3 months.
If patient fails to meet milestones (Table 3), check for Bcr-Abl mutations and switch to dasatinib 60 mg/m2 per day and initiate screening for related and unrelated allogeneic stem cell donors (would recommend switching to dasatinib rather than increasing the dose of imatinib).
If patient develops progression or relapse on dasatinib, move to myeloablative allogeneic SCT. RIC may be considered, although it is not the standard of care.
Accelerated phase CML
For children presenting with CML in accelerated phase, start dasatinib at 80 mg/m2 per day in 2 divided doses.
Initiate search for HLA-matched sibling or unrelated donors and proceed to myeloablative allogeneic SCT once in remission.
If patient fails or has a suboptimal response at any time (Table 2), proceed immediately to myeloablative allogeneic SCT.
Blast crisis CML
Start dasatinib at 80 mg/m2 per day in 2 divided doses in anti-leukemic regimen and screen for HLA-matched sibling or unrelated donors.
Once in remission, move to myeloablative allogeneic SCT.
Laboratory monitoring
Laboratory monitoring of disease burden must be performed at indicated time points or when suspicion of disease recrudescence arises (Table 7). Monitoring of CBC with differential should be performed weekly until CHR and monthly thereafter. Hematologic toxicity to TKI, manifest most commonly as either thrombocytopenia or neutropenia, typically occurs in the first 6 months of therapy. Should absolute neutrophil count fall to less than 750/μL or platelets less than 50 000/μL, then TKI should be stopped and not dose reduced. If neutropenia does not recover in 2 to 4 weeks, start filgrastim; and once the absolute neutrophil count is greater than 1000/μL, restart TKI at the same dose and taper filgrastim. Goldman40 and Kantarjian et al71 recommended monitoring for CML disease response, which includes bone marrow cytogenetic analysis every 3 to 6 months, with or without peripheral blood FISH every 3 months, with or without peripheral blood quantitative PCR every 3 months. Given the contentious treatment options (indefinite TKI vs transplantation), accurate determination of disease response is essential. Mutational analysis should be performed either when there is a rise in Bcr-Abl transcripts, loss of hematologic response, or presentation in accelerated phase or blast crisis. Even though quantitative PCR transcripts may show some variability, especially at very low absolute levels, a rise of 5- to 10-fold is considered worrisome and may prompt change in therapy.72 If there is less than a 5-fold increase in transcript levels, it is reasonable to repeat quantitative PCR in 1 to 3 months without altering therapy.72
Guidelines for laboratory monitoring of disease activity
| CBC with differential . | Every mo . |
|---|---|
| Bone marrow with cytogenetics/FISH | Every 3 mo until CCyR |
| Peripheral blood quantitative PCR for BCR-ABL | Every 3 mo until MMR, then every 6 mo |
| TKI mutation analysis | Any measure of suboptimal response: failure to reach or maintain CHR, CCyR, or MMR; rise in quantitative PCR after MMR; or increase in Ph+ chromosomes if CCyR not obtained |
| CBC with differential . | Every mo . |
|---|---|
| Bone marrow with cytogenetics/FISH | Every 3 mo until CCyR |
| Peripheral blood quantitative PCR for BCR-ABL | Every 3 mo until MMR, then every 6 mo |
| TKI mutation analysis | Any measure of suboptimal response: failure to reach or maintain CHR, CCyR, or MMR; rise in quantitative PCR after MMR; or increase in Ph+ chromosomes if CCyR not obtained |
Finally, compliance on TKI therapy is critical for patients of all age groups. Marin et al followed 87 patients with CCyR and found that medication adherence was strongly associated with the ability to obtain molecular responses.73 Similarly, the 23 patients with an adherence rate of less than or equal to 85% had a strikingly higher rate of losing their CCyR (26.8% vs 1.5%, P = .0002) compared with the 64 patients with an adherence rate of more than 85%.74 These results underscore the critical importance of compliance, often a major concern in adolescents and young adults with chronic disease. Clearly, pediatric patients need additional checks and balances to ensure compliance.
There is perhaps no other malignancy like CML where progress in biology and therapeutics has been so consistent, rapid, and transformative. Further research is needed on TKI absorption and metabolism in pediatric patients from infancy to adolescence, use of cellular therapy for minimal residual disease without prior transplantation, and stopping rules after long-term TKI therapy. Thus, it is not surprising that pediatric CML management has been and will continue to be perplexing and changing. It is time to have a global protocol and registry for pediatric CML.
Acknowledgments
This work was supported by the National Institutes of Health (grants R01CA108922, S.J.C.; and T32 CA079447, J.R.A.), and J. P. McCarthy Foundation (S.J.C.). S.J.C. was supported by Bristol-Myers Squibb.
National Institutes of Health
Authorship
Contribution: J.R.A., S.M.N., and S.J.C. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey R. Andolina, 601 Elmwood Ave, Box 777, Rochester, NY 14642; e-mail: jeffrey_andolina@urmc.rochester.edu.