Abstract
Iron supplementation strategies in the developing world remain controversial because of fears of exacerbating prevalent infectious diseases. Understanding the conditions in which iron will be absorbed and incorporated into erythrocytes is therefore important. We studied Gambian children with either postmalarial or nonmalarial anemia, who were given oral iron supplements daily for 30 days. Supplements administered on days 1 and 15 contained the stable iron isotopes 57Fe and 58Fe, respectively, and erythrocyte incorporation was measured in blood samples drawn 14 days later. We investigated how the iron-regulatory hormone hepcidin and other inflammatory/iron-related indices, all measured on the day of isotope administration, correlated with erythrocyte iron incorporation. In univariate analyses, hepcidin, ferritin, C-reactive protein, and soluble transferrin receptor (sTfR) strongly predicted incorporation of 57Fe given on day 1, while hepcidin, ferritin, and sTfR/log ferritin correlated with 58Fe incorporation. In a final multivariate model, the most consistent predictor of erythrocyte isotope incorporation was hepcidin. We conclude that under conditions of competing signals (anemia, iron deficiency, and infection), hepcidin powerfully controls use of dietary iron. We suggest that low-cost point-of-care hepcidin assays would aid iron supplementation programs in the developing world.
Introduction
Children living in poor areas with high infection rates in the developing world face conflicting challenges with respect to iron status. Iron deficiency and iron deficiency anemia are widespread,1 so that raising iron status through iron supplementation would be highly desirable, facilitating optimal cognitive and physiologic development, and alleviating the risks associated with iron-deficiency anemia.2 However, iron is also a critical mediator of host-pathogen interactions, and supplementation may have serious adverse consequences in areas of high infectious burden. Elevated iron status may increase vulnerability to bacterial,3,4 protozoal,5 and viral6 infections. Currently available data do not support definitive guidelines as to when it is safe and efficacious to administer iron in such settings, particularly in the context of malaria.7,8
The regulatory systems controlling iron absorption and localization reflect this conflict of priorities. Erythroid drive, iron deficiency, and hypoxia are all associated with increased uptake of dietary iron, while certain infections and inflammation serve to abrogate iron absorption. Extensive evidence suggests that a key molecular contributor to these effects is the liver-derived circulating peptide hepcidin, itself regulated by each of these opposing signals: it is suppressed during iron deficiency, anemia, and hypoxia, but stimulated by serum and hepatic iron, and during infection/inflammation.9-12 Hepcidin inhibits the function of ferroportin,13 the sole known mammalian iron export protein,14 expressed highly on duodenal enterocytes and iron-recycling macrophages.15-17 Therefore, when hepcidin levels are high, enterocyte absorption of dietary iron and release of macrophage iron to serum are blocked, resulting in hypoferremia that is thought to be anti-infective, but which also limits iron supply to the erythron and other tissues. It has recently been shown that the hepcidin-iron axis is a key component of innate immune defense against malarial superinfection in murine models,18 providing proof-of-principle for a likely wider role of hepcidin in protection against potentially lethal infections.
In this study, we compared the associations between erythrocyte incorporation of orally administered stable iron isotopes, hepcidin, and other indices, using samples from a previously reported study19 of iron supplementation and use in rural Gambian children with either postmalarial or nonmalarial anemia. Studies in a population such as this should be informative because several of the major stimulatory and suppressive factors directing hepcidin expression are likely to be simultaneously active. We found that hepcidin was the most consistent predictor of erythrocyte stable iron isotope incorporation in this population.
Methods
Study subjects and iron supplementation schedule
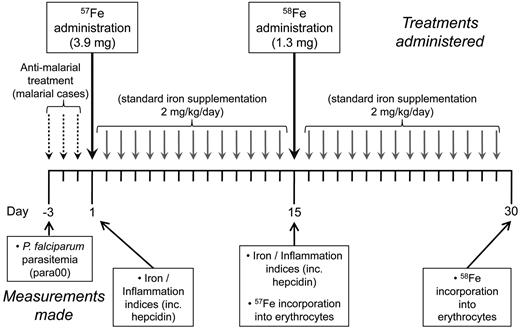
A study was previously carried out in anemic children (hemoglobin [Hb] < 110 g/L) aged 18-36 months recruited from the Medical Research Council (MRC) Keneba clinic in the West Kiang region of The Gambia during the malaria season of 2003. Children were considered as having postmalarial anemia if they presented with fever and with peripheral parasitemia (para00). Incorporation of stable iron isotopes into erythrocytes was compared between iron supplemented postmalarial anemic children (n = 37) after treatment of Plasmodium falciparum malaria (3 days of chloroquine/Fansidar, after which iron supplementation was initiated, on the day defined as “day 1, ” the fourth day after presentation with malaria) or matched anemic but nonmalarial children (n = 36), as previously described.19 Children were given a 30-day course of iron supplementation. Stable tracer isotopes consisting of non-heme 57Fe (ferrous sulfate, 3.9 mg) at day 1 and 58Fe (ferrous sulfate, 1.3 mg) at day 15 of the supplementation schedule were used, with all children receiving 2 mg/kg/d iron as liquid iron glycine sulfate on all other days of the supplementation course from day 2, as described previously.19 This schedule is depicted in Figure 1. Children were fasted for at least 2 hours before tracer dosing, which was followed by administration of 50 mg of vitamin C. These precautions minimize the influence of local inhibitory factors such as dietary phytates and facilitate iron absorption. Here, in subjects for whom sufficient residual plasma was available, we compared the relationships between hepcidin levels, other indices, and erythrocyte iron incorporation.
Schedule of malarial treatment and iron supplementation, including administration of stable iron isotopes. A course of iron supplementation was initiated in anemic Gambian children. Supplementation was initiated on day 1. Children who had presented with P falciparum parasitemia 3 days previously had their parasitemias cleared by a 3-day course of chloroquine/fansidar. The stable iron isotopes 57Fe and 58Fe were administered as sulfate on days 1 and 15, respectively. Iron, 2 mg/kg/d, was given as liquid iron glycine sulfate on all other days of the supplementation course starting from day 2.
Schedule of malarial treatment and iron supplementation, including administration of stable iron isotopes. A course of iron supplementation was initiated in anemic Gambian children. Supplementation was initiated on day 1. Children who had presented with P falciparum parasitemia 3 days previously had their parasitemias cleared by a 3-day course of chloroquine/fansidar. The stable iron isotopes 57Fe and 58Fe were administered as sulfate on days 1 and 15, respectively. Iron, 2 mg/kg/d, was given as liquid iron glycine sulfate on all other days of the supplementation course starting from day 2.
Ethics and consent
The study was approved by the Gambian government/MRC Ethics Committee and parents gave written informed consent in accordance with the Declaration of Helsinki. The parents/guardians of children were approached after attendance at the MRC Keneba clinic for either routine monitoring or after emergency treatment for malaria.
Quantification of hepcidin, erythrocyte iron incorporation, and other indices
Hepcidin concentrations in human plasma samples were quantified by competition ELISA using the hepcidin-25 (human) enzyme immunoassay kit (Bachem) according to the manufacturer's protocol. Eleven postmalarial subjects (mean age 28.1 months, SD 6.2) and 16 nonmalarial anemia subjects (mean age 28.7 months, SD 6.7) had sufficient residual plasma to assay hepcidin on days 1 and/or 15 (n = 48). Plasma samples were diluted 1 in 6 in supplied standard diluent (peptide-cleared human serum) and analyzed using a 9- or 10-point 2-fold serial dilution (maximum concentration, 25 ng/mL) standard curve. Stocks of standards, biotinylated-hepcidin tracer and anti-hepcidin primary Ab were prepared, aliquoted, and frozen in sufficient quantities to cover the complete dataset; aliquots were thawed within an hour of running each plate. Samples and standards were run in triplicate; freeze-thaw cycles were kept to a minimum. Hepcidin concentrations were interpolated from standard curves generated by logistic 4-parameter nonlinear curve fitting using Prism (Version 5.0d; GraphPad Software Inc). Samples giving readings outside the linear region of the curve were rerun at appropriate dilutions; in cases where there was a high SD between triplicate OD450 readings, samples were rerun, and the mean of all values was used in further analyses.
Hb (Medonic CA 530 hemoglobinometer) and zinc protoporphyrin (ZnPP; Aviv Biomedical hematofluorometer) were measured in The Gambia within 8 hours of sampling and each sample was assessed for the presence of malaria parasites. Frozen plasma was transported to the United Kingdom for the analysis of ferritin (Imx ferritin assay based on Microparticle Enzyme Immunoassay [MEIA] technology; Abbot Laboratories), soluble transferrin receptors (sTfR; R&D Systems ELISA), erythropoietin (EPO; R&D Systems Quantikine IVD ELISA), and C-reactive protein (CRP; Dade Dimension particle enhanced turbidimetric immunoassay). The sTfR-F index was defined as sTfR concentration divided by log serum ferritin (sTfR/log ferritin).20
For stable isotope administration, 57Fe and 58Fe were made as an aqueous solution of iron sulfate as previously described.21 Isotope ratios were measured by thermal ionization magnetic sector mass spectrometry,21,22 expressed relative to the nonadministered isotope 56Fe and corrected for temperature-specific differences in fractionation using the ratio of 54Fe to 56Fe. Iron isotope ratios were converted to tracer:tracee ratios as described23 and RBC incorporation was expressed as the percentage of dose administered.
Statistics
The distributions of variables were checked for normality and log-transformed where appropriate. Geometric means and 95% confidence intervals (CIs) are presented for these variables. Multivariate regression analysis included all variables significant at P < .05 in the univariate models. Regressions were calculated separately for the day 1, day 15, and combined data. All analysis was conducted using Datadesk 6.2.1 (Data Description Inc).
Results
Iron status and use in postmalarial and nonmalarial anemic children during a course of iron supplementation
The aim of this study was to compare the predictive value of hepcidin with those of an array of indices of iron status and inflammation (CRP, ferritin, sTfR, sTfR-F index, ZnPP, serum iron, erythropoietin, and Hb levels) for effective oral iron use, indicated by erythrocyte iron incorporation, in iron-supplemented Gambian children diagnosed with either postmalarial or nonmalarial anemia. The children with postmalarial anemia presented with fever and P falciparum parasitemias > 500 parasites/mL after Field staining of a thick blood film. They consequently completed a 3-day course of antimalarial treatment, which in every case cleared the parasitemia. Nonmalarial anemic children were identified from immunization, growth monitoring, or general clinics and otherwise appeared well; they had no documented fever in the previous 7 days, nor a record of clinical malaria episodes or antimalarial treatment during the ongoing malaria season. For all children, a 30-day course of oral iron supplementation was initiated, with postmalarial anemia cases commencing supplementation the day after completion of antimalarial treatment: this day is referred to as day 1. To investigate how effectively iron was used in each of these groups, the stable iron isotopes 57Fe and 58Fe were administered orally on days 1 and 15, respectively, and incorporation of each stable iron isotope into erythrocytes was quantified 14 days after its administration (Figure 1).
Iron indices and inflammatory markers were also measured in blood taken on the days of stable iron isotope administration. First, we compare iron status and inflammation in children from postmalarial and nonmalarial anemia groups at both day 1 and day 15 (column 2 vs column 3, and column 4 vs column 5 in Table 1), and then describe changes from day 1 to day 15 within both these groups (column 2 vs column 4, column 3 vs column 5).
Comparison of iron status and inflammation between postmalarial and nonmalarial anemic children at the initiation of iron supplementation (day 1).
On day 1, children with postmalarial anemia had significantly lower Hb levels than those in the nonmalarial anemia group (Table 1). Despite the antimalarial treatment clearing parasitemias in all postmalarial anemia cases, CRP and ferritin were significantly higher in these children than in nonmalarial anemic cases (Table 1). Likewise, hepcidin, previously shown to be induced by malaria,24-27 was raised in these children compared with nonmalarial anemic children, although not statistically significantly so (Table 1). EPO levels were significantly higher in postmalarial cases than nonmalarial anemic cases (Table 1). Serum iron levels were higher in postmalarial anemic children. The nonmalarial anemic children were more iron deficient than the postmalarial anemic children, as indicated by sTfR levels and sTfR-F index (Table 1). Erythrocyte incorporation of 57Fe, measured in blood taken 14 days after isotope administration, was significantly suppressed in postmalarial anemic children compared with the nonmalarial anemic cases, as previously described.19
Comparison of markers of iron status and inflammation between postmalarial and nonmalarial anemic children after 14 days of iron supplementation (day 15).
There were no statistically significant differences between the 2 groups of children on day 15 before administration of 58Fe. Postmalarial children had higher Hb and lower levels of EPO and ZnPP, together indicating a greater level of oxygen carriage and reduced hypoxia compared with children that were admitted to the study with nonmalarial anemia. Erythrocyte incorporation of 58Fe, measured 14 days after its administration on day 15, was less in postmalarial anemic children compared with the nonmalarial anemic cases.
Changes in iron status after 14 days of iron supplementation in postmalarial anemic children.
Despite the relative suppression of erythrocyte iron (57Fe) incorporation in postmalarial anemic children, after the first 14 days of iron supplementation Hb levels had risen notably (Table 1). Inflammation (CRP) in most postmalarial children had resolved and other hematologic indices were normalizing by this time (Table 1). Similarly, hepcidin levels had also fallen and EPO levels had dropped markedly, indicating a correction of hypoxia probably consequent on antimalarial treatment. Correspondingly, erythrocyte incorporation of 58Fe (administered on day 15) was higher than 57Fe incorporation (Table 1), suggesting the relative block to oral iron use was being relieved.
Changes in iron status after 14 days of iron supplementation in nonmalarial anemic children.
In contrast to the postmalarial anemia cases, there was no significant increase in Hb levels after the first 14 days of iron supplementation, and CRP levels remained low. However, serum iron and ferritin levels had risen by day 15, and hepcidin had risen in concert with reduced sTfR-F index (both nonsignificantly), indicative of a response to the course of iron supplementation (Table 1). The efficiency of 58Fe erythrocyte incorporation administered on day 15 was similar in these children to that of 57Fe incorporation administered on day 1, suggesting continued efficacy of oral iron supplementation throughout the course.
In summary, these data suggest that many of the conflicting signals that affect iron handling and use are represented in these groups: inflammation and recent infection, iron deficiency, anemia and erythropoietic drive. There were differences in the changes over time of iron status and inflammation in children with postmalarial anemia compared with children with nonmalarial anemia. In addition, over the course of the study, oral iron availability was maintained at a high level, so that the temporal balance of the above factors was not constant. Against this background, we wished to test how hepcidin compared with other serum indices as a predictor of iron use as measured by erythrocyte iron incorporation.
Univariate associations between erythrocyte iron incorporation, hepcidin, and other indices of iron status and inflammation
We first evaluated univariate associations between erythrocyte stable iron isotope incorporation and indices of iron status and inflammation (Table 2). Specifically, we considered associations between (1) plasma iron/inflammatory indices on the day of 57Fe administration (day 1) and subsequent 57Fe erythrocyte incorporation; (2) plasma iron/inflammatory indices on the day of 58Fe administration (day 15) and subsequent 58Fe erythrocyte incorporation; and (3) combined data for incorporation of both stable isotopes with associated plasma indices on the day of isotope administration. As expected there was significant covariation between hepcidin and ferritin (R2 = 23%, n = 48), hepcidin and CRP (R2 = 10%), and ferritin and CRP (R2 = 39%).
Predictors of 57Fe erythrocyte incorporation: assessing use of oral iron at the start of the course of iron supplementation (Table 2 left columns).
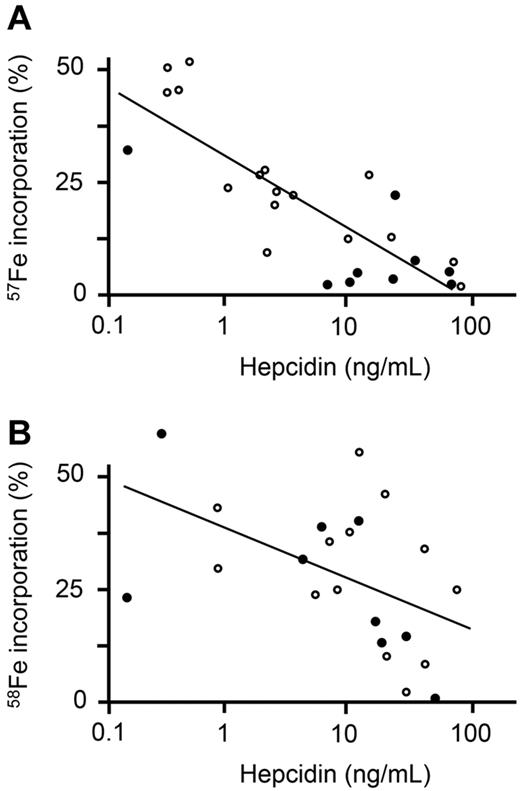
The strongest predictor of 57Fe erythrocyte incorporation was hepcidin, which was significantly negatively associated (adjusted R2 = 67%, P < .0001; Figure 2A); CRP and ferritin were also highly significant negative predictors of 57Fe incorporation. A strong positive association of 57Fe erythrocyte incorporation with sTfR, and a weaker, but significant, positive correlation with sTfR-F index were also found. These data suggest that at the start of the course of iron supplementation, there was an influence of both inflammatory stimuli and iron status on oral iron use.
Scatterplots of oral iron incorporation versus hepcidin. (A) Serum hepcidin measured on day 1 is plotted against incorporation of 57Fe (which was administered on day 1). (B) Serum hepcidin measured on day 15 is plotted against incorporation of 58Fe (which was administered on day 15). ● indicates children admitted into the study with postmalarial anemia; and ○, children admitted into the study with nonmalarial anemia. Line depicts best-fit regression.
Scatterplots of oral iron incorporation versus hepcidin. (A) Serum hepcidin measured on day 1 is plotted against incorporation of 57Fe (which was administered on day 1). (B) Serum hepcidin measured on day 15 is plotted against incorporation of 58Fe (which was administered on day 15). ● indicates children admitted into the study with postmalarial anemia; and ○, children admitted into the study with nonmalarial anemia. Line depicts best-fit regression.
Predictors of 58Fe erythrocyte incorporation: assessing use of oral iron administered 2 weeks into a 30-day course of iron supplementation (Table 2 middle columns).
Despite the strong negative correlation of CRP with 57Fe erythrocyte incorporation above, CRP did not significantly predict 58Fe erythrocyte incorporation, probably because by day 15 inflammation had waned (see Table 1). However, hepcidin remained a significant negative predictor of 58Fe incorporation (Figure 2B), suggesting it may represent a more consistent indicator of iron use. Hemoglobin, ZnPP, sTfR-F index, and ferritin were strongest predictors of 58Fe incorporation, consistent with iron status governing iron incorporation in the absence of inflammation.
Combined data (Table 2 right columns).
Taking into account erythrocyte incorporation of both 57Fe and 58Fe, the strongest predictors were CRP and hepcidin. Less significant associations were found with ferritin, sTfR, and ZnPP, but not with the sTfR-F index or Hb. There was no significant association with EPO in any of the analyses.
Multivariate analysis of factors predicting erythrocyte iron incorporation
We constructed final multivariate models including all variables displaying univariate associations with erythrocyte incorporation of either 57Fe, 58Fe, or the combined data (shown in Table 3). While ferritin dropped out of this analysis, hepcidin emerged as the most consistent predictor of the efficiency of incorporation of orally administered iron into erythrocytes: hepcidin predicted incorporation of both 57Fe administered at the start of the course of iron supplementation and 58Fe given 2 weeks into the course; it remained a significant predictor when all data were included in the final model (Table 3). CRP was also a strong predictor when all data were considered together, but despite strongly predicting 57Fe incorporation, it did not predict 58Fe incorporation. Remarkably, hepcidin, CRP, and pretreatment parasitemia (para00) explained 85% of the variance in incorporation of 57Fe, administered on day 1.
Discussion
The need to understand the conditions in which iron can be safely provided to children living in conditions of high infectious risk has become of prime public health significance8 after the serious adverse effects of supplementation observed in a large trial on Pemba Island.5
In this study, we used incorporation of orally administered stable iron isotopes into erythrocytes as a measure of effective dietary iron use in Gambian children with postmalarial or nonmalarial anemia. Other measures of iron status such as serum iron or sTfR-F index may be altered after iron supplementation, but do not necessarily equate to increased iron use for erythropoiesis. In our study population, several conflicting signals known to influence iron uptake, handling, and use are represented: inflammation, indicated by high CRP and ferritin; iron deficiency and low iron stores (low ferritin, high sTfR-F index, and high ZnPP); strong erythropoietic drive (high EPO); and increasing iron supply (because of the prescribed 30-day course of iron supplementation). Moreover, this population showed broad distributions of most of the indices measured, favoring the detection of true biologic correlates (Hb 68-133 g/L; hepcidin 0.2-94 ng/mL; CRP 0.6-121 mg/L; ferritin 1-211 ng/mL; serum iron 6-27 μmol/L; sTfR 3-34 mg/L; sTfR-F index 1-59; erythropoietin 4-196 mIU/mL; ZnPP 51-347 mmol/mol Hb; iron incorporation, 1%-68%).
Children with postmalarial anemia demonstrated blunted erythrocyte 57Fe (day 1) incorporation relative to nonmalarial anemic children as previously described.19 Despite having cleared parasitemias after 3 days of antimalarial treatment, they still had raised inflammatory markers (CRP and ferritin), presumably reflecting residual effects of malarial inflammation. Hepcidin likewise was raised in these individuals, but not as markedly as CRP or ferritin; this might either reflect differing kinetics of clearance, and/or that other hepcidin-suppresive factors were also in operation (eg, erythropoietic drive,28 hypoxia29 ). EPO levels were significantly higher in the postmalarial cases, potentially reflecting a compensatory response to malaria-mediated suppression of EPO responsiveness as previously described30-32 ; the lower Hb levels in this group could also contribute to higher EPO. Serum iron levels were raised in these children, perhaps because of residual effects of malarial hemolysis. The low levels of 57Fe incorporation despite high serum iron and the lower levels of sTfR that were also apparent despite high EPO together suggest that poor 57Fe incorporation in these children may at least in part be due to dyserythropoiesis as well as the effect of high hepcidin restricting iron availability. In malaria, inflammatory cytokines and effects of the hemozoin pigment can cause BM dysfunction. Despite this relative block to incorporation of 57Fe given on day 1, children with postmalarial anemia showed a better Hb recovery after 2 weeks of iron supplementation than nonmalarial anemic children. The rise in Hb may have been fueled by the release, after resolution of inflammation, of (non-57Fe) iron sequestered into reticuloendothelial macrophages during the acute-phase response to infection. The improved use of 58Fe, administered 2 weeks after 57Fe, is consistent with this idea. Together these factors may explain the faster Hb recovery in the face of poorer incorporation of 57Fe in this group.
Iron deficiency was present in children with nonmalarial anemia, as indicated by low serum iron, low ferritin, relatively low hepcidin, and high sTfR-F index. These children were not inflamed and incorporated dietary iron (day 1 57Fe) better than postmalarial anemic children. Furthermore, a response to 2 weeks' iron supplementation was indicated by increased serum iron, ferritin, and (nonsignificantly) hepcidin, and a nonsignificantly reduced sTfR-F index. However, the unexpected lack of change to Hb and ZnPP suggests a possible contribution of BM dysfunction, or other factors, to anemia in this group.
We next compared how hepcidin and other plasma indices measured on the day of stable iron isotope administration predicted iron incorporation into erythrocytes. Several processes influence erythrocyte incorporation of orally administered iron. Intestinal iron uptake and iron recycling are 2 key factors, and are both regulated by hepcidin-dependent and -independent mechanisms. Double-isotope studies (oral and IV) in nonpregnant Beninese women, with very similar absorption percentages to our study33 showed that 88% of absorbed iron was incorporated into erythrocytes,34 indicating the contribution of intestinal absorption to eventual RBC iron. However, iron use may also vary postabsorption–for example, iron may become trapped in macrophages, or iron may not be incorporated efficiently even if available, should erythropoiesis be inhibited (as may be the case for 57Fe incorporation in postmalarial anemia, discussed in the 2 paragraphs above).
Despite all the possible causes of variable erythrocyte iron incorporation, and the fact that these processes are not regulated by hepcidin alone, in our studies hepcidin was the most consistent predictor of subsequent erythrocyte iron incorporation, significantly correlating with incorporation of both 57Fe (administered on day 1) and 58Fe (administered on day 15), and when all isotope incorporation data were considered together. Other parameters also correlated significantly with erythrocyte iron incorporation, although not in all analyses. CRP, present at high levels immediately after the 3-day course of antimalarial treatment before commencement of iron supplementation, strongly predicted incorporation of day 1 administered 57Fe. Indeed, hepcidin, CRP, and pretreatment parasitemia explained 85% of the variance in erythrocyte incorporation of 57Fe. However, by day 15 when inflammation had subsided, CRP no longer predicted 58Fe incorporation, unlike hepcidin. This is consistent with other factors besides inflammation influencing erythrocyte iron incorporation, such that the predictive value of CRP remains limited. Ferritin (elevated by inflammation and low during iron deficiency when inflammation is absent) covaried with both hepcidin, as previously described,35 and CRP. Ferritin correlated with erythrocyte iron incorporation in univariate analyses, but was not significant in multivariate models.
Significant, but weaker, associations between hepcidin and iron absorption/use have also been reported in iron-replete European men (R2 = 30%, n = 33),36 in iron-replete nonpregnant American women (P = .03, n = 18),37 and in European women with iron status ranging from replete to moderately deficient (R2 = 31%, n = 98 from ferrous sulfate; R2 = 20%, n = 48 from ferrous fumarate; and R2 = 10% (NS) from ferric pyrophosphate).38 A study in 23 nonpregnant Beninese women with asymptomatic P falciparum parasitemia reported no association between hepcidin and iron absorption at baseline,34 but a significant association (R2 = 46%, P < .01) 25 days after treatment. The strength of the association between hepcidin and erythrocyte iron incorporation in our study may be due to a greater variance of hepcidin and hepcidin-influencing factors across the study group, and/or because we assayed erythrocyte iron rather than total body iron absorption or serum iron.
That hepcidin outperforms other signals/markers of iron and infection status (Hb, ferritin, sTfR, the sTfR-F index of body iron stores, EPO, and CRP) as a predictor of iron incorporation in this population has 2 important implications. First, it strongly suggests that hepcidin has evolved as the master regulator of host iron absorption and use in the face of infections, as previously inferred.12,39 In this respect, the fact that hepcidin causes rapid hypoferremia in response to infection39-41 and that it declines rapidly after an infection or inflammatory signal has been cleared26,27 (thus allowing rapid absorption of dietary iron and remobilization of sequestered iron) would favor safe iron acquisition by anemic children during the limited windows between repeated infections. Second, because hepcidin appears to integrate the opposing regulatory signals derived from iron deficiency and from threat of infection, it might be exploitable as an effective point-of-care index indicating “ready-and-safe to receive iron” that could guide therapeutic intervention.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the mothers and children who participated in the study. They thank The Wellcome Trust and the MRC United Kingdom for funding.
During this work, H.D. was funded by a Beit Memorial Fellowship for Medical Research and an MRC New Investigator Award.
Wellcome Trust
Authorship
Contribution: A.M.P. and C.P.D. conceived the study; C.P.D., S.A.A., and S.E.C. conducted the study and associated analyses; S.H.A. and H.V. contributed to data interpretation; A.E.A. and H.D. performed the hepcidin analyses and contributed to data interpretation; and A.M.P., A.E.A., and H.D. wrote the manuscript with input from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew M. Prentice, PhD, MRC International Nutrition Group, London School of Hygiene & Tropical Medicine, Keppel St, London, WC1E 7HT, United Kindgom; e-mail: andrew.prentice@lshtm.ac.uk.
References
Author notes
A.E.A. and H.D. contributed equally to this manuscript.