Abstract
Sickle cell anemia is characterized by chronic hemolysis coupled with extensive vascular inflammation. This inflammatory state also mechanistically promotes a high risk of lethal, invasive pneumococcal infection. Current treatments to reduce vaso-occlusive complications include chronic hydroxyurea therapy to induce fetal hemoglobin. Because hydroxyurea also reduces leukocytosis, an understanding of the impact of this treatment on pneumococcal pathogenesis is needed. Using a sickle cell mouse model of pneumococcal pneumonia and sepsis, administration of hydroxyurea was found to significantly improve survival. Hydroxyurea treatment decreased neutrophil extravasation into the infected lung coincident with significantly reduced levels of E-selectin in serum and on pulmonary epithelia. The protective effect of hydroxyurea was abrogated in mice deficient in E-selectin. The decrease in E-selectin levels was also evident in human sickle cell patients receiving hydroxyurea therapy. These data indicate that in addition to induction of fetal hemoglobin, hydroxyurea attenuates leukocyte–endothelial interactions in sickle cell anemia, resulting in protection against lethal pneumococcal sepsis.
Introduction
Sickle cell anemia (SCA) is characterized by chronic hemolytic anemia and vascular inflammation. Hydroxyurea therapy decreases vaso-occlusive complications of SCA and reduces mortality,1,2 but the mechanism of this benefit has been debated. Elevated white blood cell counts have been found to correlate with greater mortality in sickle cell disease (SCD),3 and early studies suggested hydroxyurea reduced leukocytosis.4,5 Subsequent studies found no statistical association between reduction of leukocytosis and mortality in SCA patients receiving hydroxyurea, implicating increased levels of hemoglobin F (HbF) as the main predictor of mortality.6 A recent placebo-controlled clinical trial of hydroxyurea in pediatric SCA patients confirmed a highly significant decrease in total white blood cell and absolute neutrophil counts after hydroxyurea treatment as well as increases in hemoglobin and HbF levels.7 A significant decrease in acute chest syndrome, which is oftentimes initiated by lung infection, also was observed in this study.7 Bacteremia was recorded 6 times in the placebo group but only 3 times in the hydroxyurea-treated patients,7 a trend that was not significant because of the low incidence. Although induction of HbF is generally accepted to be a major benefit of hydroxyurea therapy in SCA, the relative contribution of other factors, including decreased white blood cell counts, to positive outcomes remains a possibility that has yet to be explored.
Modulation of leukocytosis by hydroxyurea raises the question of infection risk in SCA. Children with SCA have a 400-fold greater risk of fulminant, lethal pneumococcal sepsis than their healthy peers or patients with other hemolytic anemias,8-10 a finding recapitulated in the sickle cell mouse model.11 Despite administration of penicillin prophylaxis, pneumococcal polysaccharide vaccine, and pneumococcal protein-conjugate vaccine, invasive pneumococcal disease continues to be a serious risk for patients with SCA.12 Progression of pneumococcal infection in SCD is accelerated by the widespread vascular inflammation that induces expression of endothelial receptors used for bacterial invasion.11,13,14 Recent studies have demonstrated pharmacologic reduction of vascular inflammation attenuates invasive infection.14 We sought to determine the impact of administration of hydroxyurea at a dose that reduces leukocytosis15 on the course of pneumococcal infection.
Because murine sickle cell models lack γ globin, the model also isolates effects of hydroxyurea that are independent of HbF induction.15 We demonstrate that hydroxyurea reduces lung inflammation and confers protection against pneumococcal challenge. Hydroxyurea therapy significantly reduces expression of E-selectin and decreases adhesion and extravasation of neutrophils. The proposed mechanism of protection was confirmed in E-selectin–deficient sickle cell mice that showed no additional protective benefit from hydroxyurea therapy. Patients with SCA receiving hydroxyurea therapy also were shown to have significantly reduced serum E-selectin. These data indicate that hydroxyurea affects leukocyte–endothelial interactions in SCA, resulting in protection against lethal pneumococcal sepsis.
Methods
Generation of sickle cell mice
All experiments using animals were performed with the prior approval of and in accordance with guidelines of the St Jude Institutional Animal Care and Use Committee. Lethally irradiated, 8-week-old female C57B/J6 and E-selectin–deficient (B6.129S4-Seletm1Dmil/J, stock 008236) mice (The Jackson Laboratory) were transplanted as described previously with 2 × 106 bone marrow cells from either BERK SCA mice or wild-type mice.16 Enrofloxacin (Baytril 2.27% solution; Bayer) was administered as antimicrobial prophylaxis for 3 weeks after transplantation. One hundred days after transplant, the sickle phenotype was confirmed by hemoglobin cellulose acetate electrophoresis of red cell lysates.17 Complete blood counts were determined to ascertain the white blood cell number, hematocrit, hemoglobin, and red blood cell distribution were equivalent to the sickle donor. Transplanted sickle mice were injected with 50 mg/kg hydroxyurea (Sigma-Aldrich) by intraperitoneal route 5 days/week, starting 10 weeks after transplantation, as described previously.15 This dosage was chosen based on a maximum tolerated dose experiment with the goal of obtaining a decrease in absolute neutrophil count to 2000 to 4000, similar to sickle cell patients who typically receive up to 30 mg/kg/day during continuous hydroxyurea therapy. A lower dose of 25 mg/kg/day failed to decrease the neutrophil count and was rejected. Only mice demonstrating full engraftment as confirmed by hemoglobin electrophoresis and blood counts at 12 weeks transplantation were used for further studies. Mice were used for experiments after 4 weeks of hydroxyurea administration, at 14 weeks after transplantation.
Immunohistochemistry
After euthanasia, mice were perfused with 3% paraformaldehyde after which lungs were excised and instilled intratracheal with paraformaldehyde under constant pressure to expand the lungs to approximately physiologic dimensions. For histopathology, lungs were collected at 18 hours after infection and immediately perfused with 10% formaldehyde and embedded in paraffin.
For hematoxylin and eosin (H&E) staining, tissues were embedded in paraffin, sectioned, and stained as described previously.18 Scoring of vascular inflammation was as follows: (1) rarely observed perivascular leukocytes; (2) mild infiltration of perivascular spaces with lymphocytes and plasma cells; (3) moderate perivascular infiltration of perivascular spaces, presence of lymphocytes, plasma cells, neutrophils, and focal vasculitis; and (4) marked perivascular lymphocytes, plasma cells, neutrophils, and common vasculitis. Scoring of interstitial pneumonia was as follows: (1) mild, neutrophils in alveoli and less than 5 in alveolar space; (2) moderate, greater than 5 neutrophils in alveolar spaces, foamy macrophages, some alveolar wall thickening; and (3) severe, viable and degenerate neutrophils fill alveolar spaces, alveolar walls thickened by leukocytes, foamy macrophages. Samples were scored by a veterinary pathologist unaware of the treatment group.
For antibody labeling, tissues were kept in 3% paraformaldehyde overnight. Lungs were then transferred to 30% sucrose for 3 to 5 days, frozen in OCT freezing medium (Tissue Tek; Electron Microscopy Sciences) in dry ice, and sectioned (20 μm). Sections were submersed in 0.6% hydrogen peroxide for 30 minutes, washed, permeabilized with 0.4% Triton X-100 for 20 minutes, and washed. Sections were blocked with 1% BSA and 5% goat serum for 30 minutes and incubated with primary anti–E-selectin antibody (Santa Cruz Biotechnology) at 1:300. After washing, sections were incubated with goat anti–rabbit IgG-HRP (Bio-Rad Laboratories) at 1:500 for 1 hour at room temperature. Sections were washed and visualized with VIP substrate kit and counterstained with methyl green (Vector Laboratories).
Flow cytometry
Bronchoalveolar lavage fluid was collected 6 hours after intratracheal administration of 107 ethanol-fixed pneumococci, and red blood cells were lysed using ACK lysis buffer (Lonza Walkersville Inc). Ethanol-fixed pneumococci were used to provide a consistent immune-stimulus because differential outgrowth of bacteria in the lungs can result in greater variability in the time points being studied. Single-cell suspensions were enumerated, and cells were blocked with 1% FBS and stained for surface CD11b and GR1 (eBioscience). Flow cytometry data were acquired on an FACSCalibur flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar).
Intravital fluorescence microscopy
Cranial windows were installed overlying the cerebral cortex as described previously.19,20 Mice with cranial windows were anesthetized with 1 mg of ketamine and 1 mg of xylazine in a volume of 200 μL/mouse, immobilized on a stereotaxic frame, and placed under an industrial-scale microscope (model MM-11; Nikon). One hour before surgery, mice were injected intravenously with TNFα, as described previously.14 Green fluorescent protein (GFP) neutrophils were prepared as described previously from C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ mice (stock 006567; The Jackson Laboratory).21 Video images were taken for 5 minutes after injection of GFP neutrophils using NIS Elements (Nikon). The number of adherent neutrophils was measured by an observer unaware of mouse type or treatment group.
In vivo 2-photon imaging
Two-photon laser-scanning microscopy was performed using an Ultima IV imaging system (Prairie Technologies), a Ti:sapphire Chameleon Ultra femtosecond-pulsed laser (Coherent), and 20×/0.95 numerical aperture, water-immersion infrared objectives (Olympus), as described previously.22,23 Surgery was performed over the auditory cortex, and cranial windows were installed as described under “Intravital fluorescence microscopy.” Rhodamine-dextran (10 000 MW, 1 mg/mouse; Invitrogen) was injected to visualize the vasculature along with the GFP neutrophils. Samples were excited at 900 nm, and consecutive 500 images were captured at a rate of 15 frames/second using the AOD module of the Ultima IV imaging system.
Measurement of inflammatory cytokines
Inflammatory cytokines were measured using the Milliplex map kits (Millipore) for murine and human samples. For both sample types, a CVD kit containing E-selectin, ICAM-1, and VCAM-1 was used.
Mouse challenge
Streptococcus pneumoniae strain D39x was grown on tryptic soy agar (EMD Biosciences) supplemented with 3% sheep blood or defined semisynthetic casein liquid media24,25 supplemented with 0.5% yeast extract. Hydroxyurea did not effect bacterial growth at concentrations up to 500μM. Bacterial challenge studies were performed as described previously,26 except that lethal dose (LD)50 (rather than LD100) was chosen to observe pneumonia as well as sepsis. Bacteria were introduced by intranasal administration in 25 μL of saline: 5 to 8 × 105 CFUs for transplanted mice and 2 × 106 CFUs for nontransplanted mice. The E-selectin mice were challenged with 1 × 106 CFUs in 25 μL of saline. Mice were monitored daily for signs of infection and imaged in the IVIS Imaging System 100 Series (Xenogen).27 Differences in the survival curves were calculated by Mantel-Cox log rank test. Bacterial density in blood was quantified at 24 hours after infection and compared by Mann-Whitney U test.
Hydroxyurea human protocol
Children with SCA were enrolled in the Hydroxyurea Study of Long-Term Effects (HUSTLE, NCT 00305175). Subjects in the new cohort had baseline serum samples obtained before initiating hydroxyurea treatment. The hydroxyurea dose was initially 20 mg/kg/day, but it was adjusted to reach the maximal tolerated dose (MTD) as described previously.28 Follow-up samples were obtained after reaching MTD. All blood collections and analyses using HUSTLE samples were approved by the St Jude Investigational Review Board.
Results
Impact of hydroxyurea on the course of pneumococcal infection
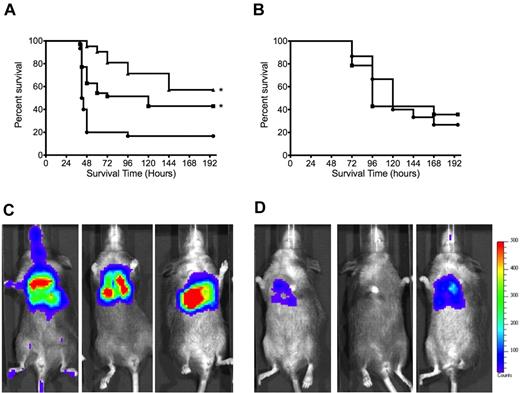
Sickle mice were treated with hydroxyurea for 4 weeks. Bacteria were administered intranasally to initiate colonization, and progression to pneumonia and hydroxyurea was continued daily after challenge. Treatment with hydroxyurea resulted in a significant delay in time to death (Figure 1A; P = .0028), and the bacterial burden in the blood was significantly (P = .01) reduced (sickle/mock median = 4.2 × 104 CFUs/mL vs sickle/hydroxyurea median below limit of detection of 103). Wild-type mice showed no protective effect of hydroxyurea therapy (Figure 1B). Xenogen imaging of the bioluminescent pneumococci were consistent with the survival and bacterial burden data with the SCA mice receiving mock treatment (Figure 1C), showing consistently greater bacterial burden than the SCA mice treated with hydroxyurea (Figure 1D).
Effect of hydroxyurea therapy on pneumococcal pneumonia. (A) Mean overall survival of SCA mice receiving hydroxyurea (squares) or saline (circles) or mock-transplanted mice receiving saline (triangles) after pneumococcal challenge (*P < .01 compared with SCD saline by Mantel-Cox log rank test). n = 31, SCA saline; n = 35, SCA hydroxyurea; n = 21, mock transplanted from at least 3 independent experiments; P = .0028 for SCA saline vs SCA hydroxyurea. No significant difference (P = .12) observed between mock-transplanted and SCD hydroxyurea groups. (B) Survival of nontransplanted wild-type mice (C57/bl6) receiving hydroxyurea (squares) or saline (circles) after pneumococcal challenge. n = 15/group (P = .93; statistical comparisons by Mantel-Cox log rank). Bacteria were introduced by intranasal administration in 25 μL of saline: 5 to 8 × 105 CFUs for transplanted mice and 2 × 106 CFUs for nontransplanted C57/bl6 mice. Representative Xenogen images of bioluminescent pneumococci indicating the progression of disease in (C) saline- and (D) hydroxyurea-treated SCA mice at 24 hours after challenge. Color intensity correlates with bacterial density (blue indicates low titer; and red, high titer).
Effect of hydroxyurea therapy on pneumococcal pneumonia. (A) Mean overall survival of SCA mice receiving hydroxyurea (squares) or saline (circles) or mock-transplanted mice receiving saline (triangles) after pneumococcal challenge (*P < .01 compared with SCD saline by Mantel-Cox log rank test). n = 31, SCA saline; n = 35, SCA hydroxyurea; n = 21, mock transplanted from at least 3 independent experiments; P = .0028 for SCA saline vs SCA hydroxyurea. No significant difference (P = .12) observed between mock-transplanted and SCD hydroxyurea groups. (B) Survival of nontransplanted wild-type mice (C57/bl6) receiving hydroxyurea (squares) or saline (circles) after pneumococcal challenge. n = 15/group (P = .93; statistical comparisons by Mantel-Cox log rank). Bacteria were introduced by intranasal administration in 25 μL of saline: 5 to 8 × 105 CFUs for transplanted mice and 2 × 106 CFUs for nontransplanted C57/bl6 mice. Representative Xenogen images of bioluminescent pneumococci indicating the progression of disease in (C) saline- and (D) hydroxyurea-treated SCA mice at 24 hours after challenge. Color intensity correlates with bacterial density (blue indicates low titer; and red, high titer).
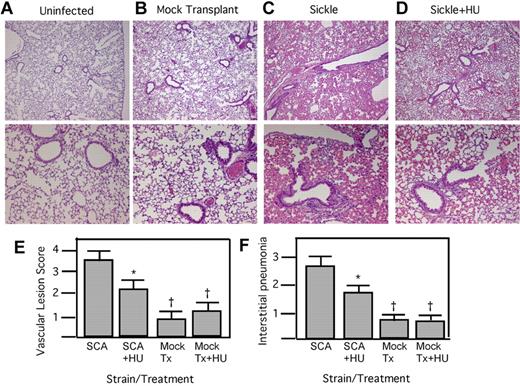
Histopathologic findings in the lungs were consistent with survival data. In the mock-treated sickle animals, 80% showed severe interstitial pneumonia characterized by neutrophils filling the alveolar space, thickened alveolar walls, and the presence of foamy macrophages (Figure 2C). The vessels contained marked perivascular lymphocytes, plasma cells, sickled erythrocytes, and neutrophils. Vasculitis was common in more than 80% of the sections examined. In contrast, 80% of hydroxyurea-treated animals displayed modest interstitial pneumonia with few neutrophils in the alveolar space and less alveolar wall thickening (Figure 2D). The vascular lesions were more moderate in comparison with the mock-treated animals, with less perivascular infiltration by lymphocytes. Quantitation of the histopathologic signs of interstitial pneumonia and vasculitis showed significant reduction in the SCA, mock-transplanted mice undergoing hydroxyurea therapy (Figure 2E-F). Wild-type mice showed no changes in lung inflammation in response to hydroxyurea (Figure 2E-F). These data indicate that sickle mice receiving hydroxyurea therapy have significantly reduced lung inflammation and damage in response to bacterial infection and improved survival.
Histopathology of SCA lungs after pneumococcal challenge. (A-D) H&E-stained lung sections at 18 hours after infection: control without infection (A); mock transplanted, infected, saline treated (B); SCA, infected, saline treated (C); and SCA infected receiving hydroxyurea (HU; D). Magnification: top row, ×4; bottom row, ×10. (E) Mean vascular lesion scores of SCA and mock-transplanted (Tx) mice receiving HU or saline therapy at 24 hours after challenge (n = 5/group). (F) Quantification of the signs of interstitial pneumonia determined by histologic examination of lung sections at 24 hours after infection (n = 5 mice/group; mean = 3.7 ± 0.5 for SCA saline, 2.6 ± 0.4 for SCA + HU; *P < .05 compared with SCA, †P < .05 compared with SCA+HU using Mann-Whitney).
Histopathology of SCA lungs after pneumococcal challenge. (A-D) H&E-stained lung sections at 18 hours after infection: control without infection (A); mock transplanted, infected, saline treated (B); SCA, infected, saline treated (C); and SCA infected receiving hydroxyurea (HU; D). Magnification: top row, ×4; bottom row, ×10. (E) Mean vascular lesion scores of SCA and mock-transplanted (Tx) mice receiving HU or saline therapy at 24 hours after challenge (n = 5/group). (F) Quantification of the signs of interstitial pneumonia determined by histologic examination of lung sections at 24 hours after infection (n = 5 mice/group; mean = 3.7 ± 0.5 for SCA saline, 2.6 ± 0.4 for SCA + HU; *P < .05 compared with SCA, †P < .05 compared with SCA+HU using Mann-Whitney).
Effect of hydroxyurea on leukocytes
Consistent with previous studies of hydroxyurea in both patients and murine models, a significant decrease in the number of circulating leukocytes, particularly neutrophils, was observed after hydroxyurea therapy (mean = 6.1 vs 4.7 × 103 cells/μL; P = .003). To ascertain the degree to which neutrophils could be recruited to the lung in response to a defined, nonreplicating inflammatory stimulus, killed pneumococci were administered intratracheally to mock- or hydroxyurea-treated sickle mice, and lung lavages were collected 6 hours after challenge. A significant (P < .05, Mann-Whitney) reduction in the number CD11b+/GR1+-positive neutrophils was seen in the lungs of the hydroxyurea-treated animals compared with the mock group (Figure 3A; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To examine neutrophil adherence to the endothelia as a critical step in recruitment, GFP neutrophils were injected into sickle mice fitted with cranial windows to allow visual monitoring of adherent neutrophils in real time. Although SCA mice showed numerous neutrophils adherent to vessels (Figure 3B-C; supplemental Video 1), the hydroxyurea-treated animals had significantly (P < .05, Mann-Whitney) fewer adherent neutrophils (Figure 3B,D; supplemental Video 2). Similar observations were made when imaged using 2-photon microscopy whereby adherence and extravasation of the neutrophils were more apparent in the SCA mock animals (Figure 3E; supplemental Video 3) compared with the SCA animals treated with hydroxyurea (Figure 3F; supplemental Video 4). This indicates that the effect of hydroxyurea in the SCA mice was apparent at the level of neutrophil adherence and margination to endothelia and resulted in decreased pulmonary leukocytosis.
HU attenuates neutrophil adhesion and migration. (A) Neutrophil recruitment to the lung in response to killed pneumococci administered intratracheally was quantitated by FACS of bronchoalveolar lavage from saline- and HU-treated SCA mice 6 hours after challenge (n = 6 mice/group; PBS 26.2 ± 16.8, HU = 5.16 ± 3.00; * P < .05 compared with SCA by Mann-Whitney). Data represent percentage of total cells isolated from bronchoalveolar lavage. (B) SCA mice ± HU were exposed to intravenous TNFα to induce endothelial activation and neutrophil margination. Neutrophil adherence was quantitated in cranial windows (5 minutes at ×40 per mouse for at least 4 mice/group; *P < .05 compared with SCA by Mann-Whitney). (C-D) Representative images through cranial windows on SCA (C) or SCA + HU (D) mice. Neutrophils indicated by arrows, ×10 magnification. (E-F) Representative images of GFP neutrophils (green) imaged by 2-photon microscopy in the rhodamine-dextran–labeled vasculature (red) of SCA mice (E) and SCA mice treated with HU (F).
HU attenuates neutrophil adhesion and migration. (A) Neutrophil recruitment to the lung in response to killed pneumococci administered intratracheally was quantitated by FACS of bronchoalveolar lavage from saline- and HU-treated SCA mice 6 hours after challenge (n = 6 mice/group; PBS 26.2 ± 16.8, HU = 5.16 ± 3.00; * P < .05 compared with SCA by Mann-Whitney). Data represent percentage of total cells isolated from bronchoalveolar lavage. (B) SCA mice ± HU were exposed to intravenous TNFα to induce endothelial activation and neutrophil margination. Neutrophil adherence was quantitated in cranial windows (5 minutes at ×40 per mouse for at least 4 mice/group; *P < .05 compared with SCA by Mann-Whitney). (C-D) Representative images through cranial windows on SCA (C) or SCA + HU (D) mice. Neutrophils indicated by arrows, ×10 magnification. (E-F) Representative images of GFP neutrophils (green) imaged by 2-photon microscopy in the rhodamine-dextran–labeled vasculature (red) of SCA mice (E) and SCA mice treated with HU (F).
Hydroxyurea decreases E-selectin
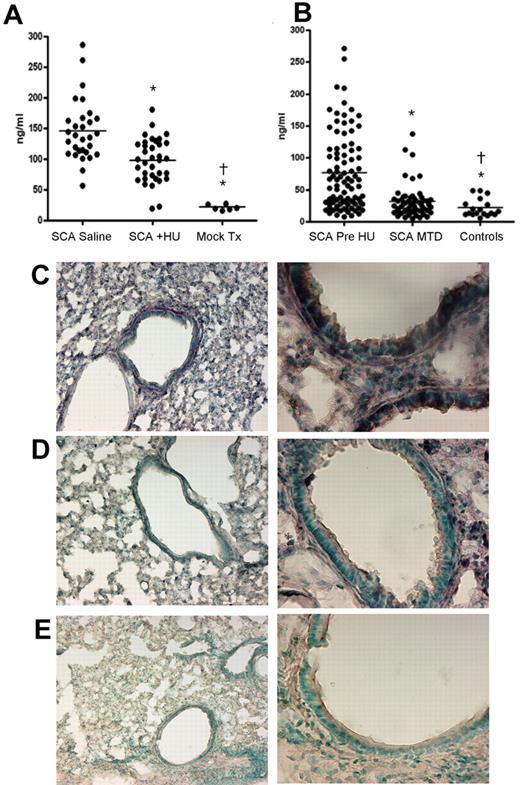
Several host receptors are required for the binding and recruitment of neutrophils to the site of infection, including E-selectin and ICAM-1. We sought to determine whether any markers of vascular inflammation were altered in sickle mice with or without hydroxyurea therapy. We observed a significant (P < .001) decrease in the amount of soluble E-selectin in the serum of hydroxyurea-treated sickle mice (Figure 4A) but no significant differences in soluble ICAM-1 or VCAM-1 levels between these 2 groups (supplemental Figure 2). Furthermore, we did not observe any significant decreases in the amount of soluble inflammatory cytokines, including TNFα, IL-6, IL-1β, or IL-12 (data not shown). The decreased amount of E-selectin also was observed in the lungs of sickle mice treated with hydroxyurea. Untreated SCA mice displayed high levels of E-selectin on pulmonary epithelia, underscoring the heightened pulmonary inflammation (Figure 4C). Hydroxyurea treatment reduced the amount of E-selectin found throughout the lung in 80% of the animals examined (Figure 4D), indicating that soluble E-selectin levels correlated well with decreased expression on the cellular surface. No discernible differences were observed for platelet activating factor receptor (PAFr) or P-selectin expression in the lung sections (data not shown).
Effect of HU on E-selectin levels in lung and serum. (A) Measurements of soluble E-selectin in the serum of mice (SCA saline, n = 30, mean = 145 ± 45 ng/mL; SCA + HU, n = 35, mean = 98.5 ± 35.5 ng/mL). (B) Measurements of soluble E-selectin in the serum of humans (SCA/pre-HU, n = 92, mean = 77 ± 60; SCA/MTD, n = 57, mean = 31 ± 26; healthy controls, n = 18, mean = 22 ± 14) undergoing HU therapy. All groups significantly different (P < .001) from all other groups by 2-tailed t test (*compared with SCD mock; †compared with SCD + HU). (C-E) Representative sections of lung stained for E-selectin (purple) from SCA animals receiving saline (C), HU (D), or a negative control with no primary antibody (E). Magnification: left column, ×4; right column, ×10.
Effect of HU on E-selectin levels in lung and serum. (A) Measurements of soluble E-selectin in the serum of mice (SCA saline, n = 30, mean = 145 ± 45 ng/mL; SCA + HU, n = 35, mean = 98.5 ± 35.5 ng/mL). (B) Measurements of soluble E-selectin in the serum of humans (SCA/pre-HU, n = 92, mean = 77 ± 60; SCA/MTD, n = 57, mean = 31 ± 26; healthy controls, n = 18, mean = 22 ± 14) undergoing HU therapy. All groups significantly different (P < .001) from all other groups by 2-tailed t test (*compared with SCD mock; †compared with SCD + HU). (C-E) Representative sections of lung stained for E-selectin (purple) from SCA animals receiving saline (C), HU (D), or a negative control with no primary antibody (E). Magnification: left column, ×4; right column, ×10.
To ascertain whether hydroxyurea attenuation of E-selectin expression was operative in human SCA patients receiving hydroxyurea therapy, serum samples collected before therapy and at MTD were analyzed for levels of soluble E-selectin, ICAM-1, and VCAM-1. In excellent agreement with the murine data, we observed a significant (P < .001) decrease in E-selectin levels after hydroxyurea treatment in patients (Figure 4B) and no significant differences in either ICAM-1 or VCAM-1 (data not shown).
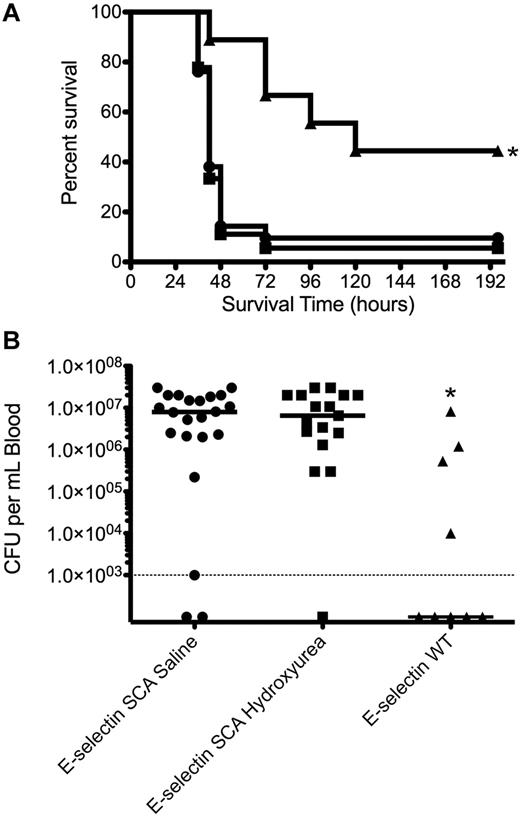
To confirm that the protective benefit of hydroxyurea in the murine SCA model was indeed dependent on E-selectin, we next undertook experiments in which E-selectin–deficient mice were engrafted with SCA bone marrow, generating an E-selectin–deficient SCA mouse. We recognize that E-selectin–deficient mice have poor leukocyte recruitment and fail to clear bacteria, resulting in increased sensitivity to systemic pneumococcal infection.29,30 However, even though the baseline survival is lower in E-selectin–deficient mice than in wild-type mice (with and without transplant to SCA), improved survival of the SCA E-selectin–deficient mice by hydroxyurea would indicate independence from E-selectin. The mice underwent hydroxyurea or saline therapy as described for the mice in Figure 1A, and they were subsequently challenged with pneumococci. Hydroxyurea was found to confer no significant protective benefit in SCA mice in the absence of E-selectin at either the level of mean time to death, overall survival (Figure 5A), or bacterial invasion into the blood (Figure 5B). These data indicate that attenuation of the heightened levels of E-selectin in SCA mice is associated with improved survival.
Lack of effect of hydroxyurea on pneumococcal pneumonia in E-selectin deficient SCA mice. (A) Mean overall survival of E-selectin–deficient (triangles; n = 9; WT indicates wild-type), E-selectin–deficient/SCA mice treated with saline (circles; n = 21), and E-selectin–deficient/SCA mice treated with hydroxyurea (squares; n = 18). Mice were challenged with 3 × 106 CFUs in 25 μL saline. No significant difference (Mantel-Cox log rank test, P = .744) was observed between the E-selectin–deficient/SCA mice untreated versus treated with hydroxyurea. (B) Log CFUs/mL of bacteria in blood of mice in panel A at 24 hours after challenge. Each symbol is a mouse, and the bar is the mean. — indicates limit of detection. The asterisk indicates a statistically significant difference compared with SCA saline group (P = .03 for survival, P = .003 for blood titers).
Lack of effect of hydroxyurea on pneumococcal pneumonia in E-selectin deficient SCA mice. (A) Mean overall survival of E-selectin–deficient (triangles; n = 9; WT indicates wild-type), E-selectin–deficient/SCA mice treated with saline (circles; n = 21), and E-selectin–deficient/SCA mice treated with hydroxyurea (squares; n = 18). Mice were challenged with 3 × 106 CFUs in 25 μL saline. No significant difference (Mantel-Cox log rank test, P = .744) was observed between the E-selectin–deficient/SCA mice untreated versus treated with hydroxyurea. (B) Log CFUs/mL of bacteria in blood of mice in panel A at 24 hours after challenge. Each symbol is a mouse, and the bar is the mean. — indicates limit of detection. The asterisk indicates a statistically significant difference compared with SCA saline group (P = .03 for survival, P = .003 for blood titers).
Discussion
Hydroxyurea is increasingly used to treat patients with SCA to decrease the complications of anemia and microcirculatory vasculitis by inducing fetal hemoglobin. This study focuses on the HbF-independent effects of hydroxyurea that seem to play a significant role in decreasing the heightened susceptibility to invasive pneumococcal infection in this population. We demonstrate that hydroxyurea improved survival of SCA mice in a model of pneumonia and sepsis. Treatment induced a systemic decrease in neutrophil adhesion to activated endothelia that resulted in decreased neutrophil recruitment to the infected lung and significantly attenuated lung damage. There are several possible mechanisms for this effect.
Both the pulmonary and systemic vascular response to a variety of inflammatory stimuli is exacerbated in SCA mice.31 The fulminant course of pneumococcal infection in SCA derives, at least in part, from this vascular inflammation. Inflammation up-regulates receptors on endothelial cells, including the PAFr that is used by pneumococci to translocate across epithelia and endothelia as invasive disease progresses.13 Thus, the deterioration of pneumonia to bacteremia is accelerated in SCD mice in a PAFr-dependent manner, and anti-inflammatory therapy, such as statins, decreases PAFr expression and slows disease progression.14 However, hydroxyurea was shown in this study not to affect PAFr expression, suggesting that the mechanism of protection must be different.
Recent evidence has clarified the mechanistic reason for the severity of systemic pneumococcal disease.32 Functional asplenia is operative both in murine and human SCA, but hydroxyurea only improves spleen function when HbF levels are increased.15,33 The lack of HbF in the SCA murine model exposes the HbF-independent effects of hydroxyurea and thus focuses on the benefit of hydroxyurea independently of the spleen. In human patients, the induction of HbF and improved spleen function would potentially add to the protective benefit that we have modeled in the murine system.
Another major player determining the progression of any infection is the leukocyte response. SCA is characterized by a circulating leukocytosis and anti-inflammatory strategies that reduce the adhesion of leukocytes improve the microcirculatory pathophysiology of SCA mice.34,35 Clinical studies have indicated that cytotoxic agents, such as hydroxyurea, not only increase HbF production but also decrease leukocytosis because of myelosuppression of the bone marrow.4,15 Although hydroxyurea reduces the incidence of vaso-occlusive events in SCA,5,36,37 the impact on mortality or rate of infection has not been well characterized. There is a suggestion that patients receiving hydroxyurea therapy may have reduced pulmonary disease.6,7 We demonstrate that hydroxyurea not only decreased circulating leukocytosis but also strongly reduced leukocyte recruitment to the infected lung that was associated with significant protection from lung damage, reduced invasion of bacteria into the bloodstream, and improved survival from pneumococcal pneumonia in the mouse SCA model. This is consistent with the concept that although leukocytes are required to clear infection, high numbers of leukocytes can damage host tissues and lead to poor outcome despite bacteriologic cure.38,39
The possible underlying mechanism for the effect of hydroxyurea on leukocyte recruitment was revealed to involve endothelial leukocyte adhesion molecules. Various adhesion molecules, including ICAM-1, VCAM, P-selectin, and E-selectin, are elevated in SCA patients.40 Hydroxyurea specifically decreased the expression of E-selectin on sickle mouse pulmonary epithelial and vascular endothelial cells. This effect was not observed for ICAM-1 or VCAM, similarly to previous findings in human SCA patients.41 We show that this mechanism was also evident in children with SCA, where hydroxyurea decreased basal leukocytosis and sE-selectin levels in serum. Sickle monocytes induce significantly higher levels of E-selectin in endothelial cells compared with monocytes from healthy controls.42 After hydroxyurea therapy in SCA patients4 as well as in mice, the number of circulating monocytes is reduced consistent with decreased sE-selectin, suggesting a potential mechanism for the observations of this study.
The recently completed pediatric phase 3 clinical trial of hydroxyurea (BABY HUG, clinicaltrials.gov, NCT 00006400) tested the clinical safety and efficacy of hydroxyurea therapy for infants with SCA. The incidence of sepsis and bacteremia was lower for infants receiving hydroxyurea (5 participants in placebo arm vs 2 participants in hydroxyurea arm; P = .26) but not statistically different in the BABY HUG trial.7 With a low incidence of pneumococcal infection, a larger sample size is required to power future studies evaluating the clinical efficacy of hydroxyurea in preventing pneumococcal sepsis. Recognizing the clinical safety of hydroxyurea in this setting and the potential to reduce the risk of pneumococcal sepsis suggested by the mouse SCA model should further add to the accumulating evidence that suggests hydroxyurea is a treatment of choice for patients with SCA.43 Pharmacologic targeting of inflammation to alleviate vaso-occlusive complications in SCA has attracted considerable attention and numerous therapies have shown great promise.34,44-46 Our data suggest that the decrease in leukocyte adhesion and migration in response to hydroxyurea therapy of SCA attenuates the host inflammatory status independent of HbF expression and has profound beneficial effects on the outcome of pneumococcal pneumonia. The observation that a similar decrease in sE-selectin was observed both in murine and human SCA subjects is encouraging. Because multiple approaches for reducing infection risk seem to have distinct mechanisms of action in the SCA host, there exists the exciting possibility that such therapies may have an additive effect and further reduce susceptibility to infection.11,14
There is an Inside Blood commentary on this article in this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Animal Imaging Center for technical assistance with the cranial windows and Justin Burton for technical assistance with the injections. The Cell Sorting Core performed the FACS.
This work was supported by grants R01 HL-090941 (R.E.W.), U54 HL-070590 (R.E.W., D.A.P., E.I.T.), R01 AI27913 (E.I.T.), R01 MH-079079 (S.S.Z.), and F32 AI082888 (J.W.R.); U54 HL-070590 Sickle Scholar Award (J.W.R.) from the National Institutes of Health; and the American Lebanese Syrian Associated Charities.
National Institutes of Health
Authorship
Contribution: J.D.L., S.S.Z., E.I.T., D.A.P., and J.W.R. designed the experiments; T.H. and R.E.W. collected and analyzed the human samples; J.D.L., T.I.P., G.G., M.J., S.S.Z., and J.W.R. performed the animal experiments; Y.H. performed the histology; and all authors assisted in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.D.L. is Division of Pediatric Hematology and Oncology, University of Alabama at Birmingham, Birmingham, AL.
Correspondence: Jason W. Rosch, 262 Danny Thomas Pl, MS 320, Memphis, TN 38103; e-mail: jason.rosch@stjude.org.
References
Author notes
J.D.L. and J.W.R. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal