Hydroxyurea treatment of sickle cell mice improved their survival from pneumococcal pneumonia, counteracting the abnormally elevated inflammatory response and reducing invasion of bacteria into the bloodstream, through down regulation of E-selectin.
Why was this study conducted in an animal model? Why use mouse models to examine major clinical issues of pneumococcal infection and hydroxyurea in sickle cell disease (SCD), well-traveled roads of investigation with abundant clinical data? In this issue of Blood, Lebensburger and colleagues make elegant use of genetically modified mice to investigate mechanisms of SCD pathophysiology and mechanisms of therapy.1 New hypotheses generated with the animal data were then tested with analysis of clinical samples, providing a fine example of the utility of animal models and translational research.
Two seemingly contradictory findings in SCD form the background of this study. First, it is well known that the natural history of SCD includes a 400-fold higher death rate from pneumococcal sepsis, suggesting that the immune response in SCD is deficient. Newborn screening, prophylactic antibiotics, and immunizations against pneumococcus have dramatically modified this natural history, but children with SCD remain very susceptible to pneumococcal disease.2 Second, SCD manifests a baseline condition of heightened inflammation, including abnormally elevated white blood cell counts, abnormally adherent blood cells, and abnormal elevation of cytokines and inflammatory mediators such as E-selectin and P-selectin.3-5 Furthermore, elevations of all inflammatory parameters generally occur with acute illness in human SCD.
This topic clearly calls for animal model investigation. The incidence of invasive pneumococcal infections in SCD patients is so low that a clinical trial of these unpredictable clinical events would be a very large and expensive study. Administering standardized experimental infection to humans with SCD would be unethical. Finally, studies of host response to infection require a whole organism, and thus cannot be conducted in cell culture or mathematical models. The Berkley mouse model of severe SCD showed that a mouse model of SCD has an exaggerated response to inflammatory challenge.6
Lebensburger et al begin by acknowledging that this mouse model of SCD lacks γ globin, offering the opportunity to isolate the effects of hydroxyurea independent of HbF induction.1 Other hydroxyurea beneficial effects through elevation of HbF can be seen in humans.
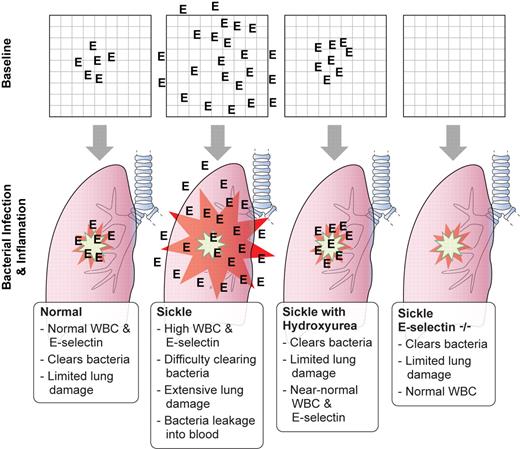
Lebensburger and colleagues generated subjects for their first experiments by transplantation of sickle mouse marrow or wild-type mouse marrow into wild-type mice. Hydroxyurea therapy lowered the neutrophil count to levels similar to human SCD patients on hydroxyurea therapy. When Streptococcus pneumonia bacteria were inoculated into the airways, the hydroxyurea-treated sickle mice had significantly better survival than saline-treated sickle mice (as shown in Figure 1A in Lebensburger et al1 ). Imaging studies with bioluminescent pneumococci show lower bacterial burden in the hydroxyurea-treated sickle mice. Lung histopathology and neutrophil adherence studies bolster the evidence of an attenuated response in the hydroxyurea-treated sickle mice. A significant decrease in E-selectin was found in the serum of hydroxyurea-treated sickle mice (demonstrated in Figure 4A in Lebensburger et al1 ). E-selectin was highly expressed in the lungs of sickle mice, and hydroxyurea treatment reduced E-selectin expression by 80% (see figure).
Baseline expression of E-selectin (represented by the frequency of “E” in each panel) affects the outcome of bacterial pneumonia challenge with Streptococcus pneumoniae. Professional illustration by Kenneth X. Probst.
Baseline expression of E-selectin (represented by the frequency of “E” in each panel) affects the outcome of bacterial pneumonia challenge with Streptococcus pneumoniae. Professional illustration by Kenneth X. Probst.
The next experiment examined whether the lowering of E-selectin is the mechanism for the protective benefit of hydroxyurea. Experimental subjects were generated by transplanting sickle marrow into E-selectin–deficient mice. The authors reason that improved survival of sickle cell E-selectin–deficient mice by hydroxyurea treatment would indicate independence from E-selectin. In the paper, Figure 5 shows that hydroxyurea confers no protective benefit in sickle mice deficient in E-selectin. Lebensburger et al concluded that attenuation of the heightened levels of E-selectin in sickle mice is associated with improved survival.1
Their final experiment used clinical SCD blood samples obtained during the Pediatric Hydroxyurea Phase III Clinical (BABY HUG) randomized trial.7 The E-selectin levels were elevated in SCD patients before hydroxyurea, and significantly lower when the patients were on hydroxyurea (Figure 4B in Lebensburger et al1 ). Additional supporting evidence from the BABY HUG trial is that fewer infections occurred on the hydroxyurea arm than the placebo group, although it was not statistically significant.
The limitations of the study include examination of just one type of bacterial infection, and the species differences in immune response. The reader should note that cross-comparison of survival fractions in the Kaplan-Meier survival curves in the study shown in Figures 1A and B and 5 is not appropriate because each figure represents a different bacterial dose.1 This is an experimental design that conserved animals because the detection of survival differences is optimized near the LD50. Likewise, lung lavage experiments were conducted with killed bacteria in Figure 3A and B, and neutrophil adherence and migration studies were conducted with TNFa as the stimulus in Figure 3C through F.1 Neutrophil adherence and migration studies were performed in a cranial window microcirculatory preparation even though the focus of the paper is pneumonia—presumably this technique was chosen because visualization of lung microcirculation has major technical challenges (tissue motion, edema, high mortality).
Thus, Lebensburger and coauthors reconcile the seemingly contradictory clinical features of increased pneumococcal susceptibility and heightened inflammatory response in SCD by proposing that the damaged, inflamed lung might provide portals for bacteria to enter the blood stream and increase the lethality of sepsis.1 In addition, lung tissue damage can aggravate local hypoxia and sickle red cell deformation, which could propagate pneumonitis quickly in sickle cell acute chest syndrome. There is some parallel to bacterial pneumonia after influenza: a state of heightened inflammation, while perhaps helpful for clearing an infection, also causes more tissue damage in the lungs and increases susceptibility to bacterial superinfection.8
This translational study advances the field and raises more questions: (1) Will the trend toward fewer infectious complications in the hydroxyurea arm of the BABY HUG study of young children with SCD be confirmed in other hydroxyurea clinical trials? Or is this an additional impetus for a registry of hydroxyurea patients? (2) Will other anti-sickling therapy also attenuate the inflammatory response in SCD to a more appropriate level? (3) How many other human diseases have a similar need for attenuation of inflammatory response?
One conceptual framework for inflammation in SCD might be an excess of yang over ying and disruption of homeostasis, but perhaps the Goldilocks Principle is more applicable. In the children's story of Goldilocks and the 3 bears, Goldilocks searches for the bowl of porridge that is “not too hot, not too cold, but just right.” Lebensburger and colleagues indicate that SCD inflammation is “too hot,” but hydroxyurea attenuates inflammation to make it “just right.”
Conflict-of-interest disclosure: The author has been an investigator and consultant for a clinical trial of an inhibitor of all Selectins (GMI-1070, Glycomimetics) in sickle cell pain. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal