Abstract
The relationship between low-density lipoprotein receptor–related protein-1 (LRP1) and von Willebrand factor (VWF) has remained elusive for years. Indeed, despite a reported absence of interaction between both proteins, liver-specific deletion of LRP1 results in increased VWF levels. To investigate this discrepancy, we used mice with a macrophage-specific deficiency of LRP1 (macLRP1−) because we previously found that macrophages dominate VWF clearance. Basal VWF levels were increased in macLRP1− mice compared with control mice (1.6 ± 0.4 vs 1.0 ± 0.4 U/mL). Clearance experiments revealed that half-life of human VWF was significantly increased in macLRP1− mice. Ubiquitous blocking of LRP1 or additional lipoprotein receptors by overexpressing receptor-associated protein in macLRP1− mice did not result in further rise of VWF levels (0.1 ± 0.2 U/mL), in contrast to macLRP1+ mice (rise in VWF, 0.8 ± 0.4 U/mL). This points to macLRP1 being the only lipoprotein receptor regulating VWF levels. When testing the mechanism(s) involved, we observed that VWF-coated beads adhered efficiently to LRP1 but only when exposed to shear forces exceeding 2.5 dyne/cm2, implying the existence of shear stress-dependent interactions. Furthermore, a mechanism involving β2-integrins that binds both VWF and LRP1 also is implicated because inhibition of β2-integrins led to increased VWF levels in control (rise, 0.19 ± 0.16 U/mL) but not in macLRP1− mice (0.08 ± 0.15 U/mL).
Introduction
von Willebrand factor (VWF) is a hemostatic protein, the physiologic relevance of which is illustrated by the severe bleeding tendency associated with its functional deficiency. The contribution of VWF to hemostasis is 2-fold: (1) VWF is essential for the recruitment of platelets to the damaged vessel wall, particularly under conditions of arterial shear; and (2) VWF functions as a carrier protein for factor VIII (FVIII), a protein cofactor critical to the coagulation system.
Whereas biosynthesis and secretion of VWF have been subject of study for more than 35 years, it is only in the last decade that clearance mechanisms of VWF have gained attention.1,2 This has led to the discovery that increased clearance of VWF may explain part of the reduced VWF levels in von Willebrand disease (VWD).3–6 This seems to be most prominent in case of VWD-type 1, although VWD-type 2 variants also are associated with reduced survival of the mutated VWF molecules.2,7,8 The increased attention is further related to the development of novel therapeutic FVIII concentrates that are used in the treatment of hemophilia A. Given that FVIII circulates in a tight complex with VWF, it is already known that VWF is a major determinant of FVIII clearance. Indeed, the half-life of FVIII is considerably reduced in patients lacking VWF antigen or in those in which VWF is unable to bind FVIII correctly (VWD-type 2N).9,10 Moreover, preinfusion VWF levels are positively correlated with FVIII half-life.11,12 To this end, we have recently demonstrated that the half-life of FVIII can be fairly well predicted using blood group and the ratio of VWF propeptide over VWF antigen (a surrogate marker for VWF clearance) as parameters.13 Thus, understanding VWF clearance is necessary to understand clearance of FVIII.
At present, there is limited information about the molecular mechanisms responsible for the clearance of VWF. As for the identity of tissues that contribute to VWF clearance, we have demonstrated previously that the liver takes up the bulk of VWF, although other organs such as the spleen also are able to remove VWF from the circulation.5 In search for the cells that are responsible for this process, we have been able to identify macrophages as being efficient in the uptake of VWF both in vitro and in vivo.14 Indeed, immunohistochemical analysis of liver and spleen tissue sections of VWF-deficient mice treated with human VWF revealed a clear colocalization of VWF with liver and spleen macrophages, respectively. Furthermore, GdCl3-mediated depletion of macrophages resulted in a 2-fold rise in endogenous VWF levels and an almost 2-fold increase in survival of VWF.14
Despite the progress made in our understanding of VWF clearance in the last decade, we are actually unaware of the receptors that contribute to the uptake of VWF in these macrophages. In contrast, for FVIII it has been reported that a liver-specific deletion of low-density lipoprotein (LDL) receptor–related protein-1 (LRP1) results in a delayed clearance of FVIII and increases endogenous FVIII levels.15 This seems surprising, because binding of intact FVIII to LRP1 is inhibited by VWF.16,17 The possibility exists that LRP1 contributes to the clearance of free FVIII (∼ 2%-5% of all FVIII molecules) and leaves VWF-bound FVIII unaffected. Another option is that LRP1 modulates VWF clearance. Indeed, these mice with a liver-specific deficiency of LRP1 also are characterized by a 2-fold increase in endogenous VWF levels,15 suggesting a potential link between LRP1 and VWF plasma levels. LRP1 is a multiligand receptor, recognizing more than 40 different ligands.18 Furthermore, LRP1 may form heterologous receptor complexes, thereby promoting the function of its partner; examples include the PDGF receptor on smooth muscle cells and the N-methyl-d-aspartate receptor on neuronal cells.19–21 Interestingly, LRP1 also is able to partner with leukocyte-specific β2-integrins.22–24 Moreover, we found that these β2-integrins can interact with VWF, providing a potential bridge between LRP1 and VWF.25
Knowing that macrophages dominate the clearance of VWF, we have used a mouse model in which LRP1 is specifically deleted in the macrophages. Using this model, we revealed that the absence of macrophage LRP1 is associated with increased levels of VWF as well as a prolonged half-life of this protein. Additional studies revealed that this LRP1-dependent effect could originate from reduced interactions between VWF and β2-integrins as well as from direct, shear stress-dependent interactions between VWF and LRP1. Altogether, we have identified LRP1 as a modulator of VWF clearance.
Methods
Mice
Three mouse strains were used, all of which being on a C57BL/6J background: FVIII-deficient mice were purchased from The Jackson Laboratory, and LRP1flox/flox (macLRP1+) and LRP1flox/flox/LysM-Cre+ (macLRP1−) have been described previously.26–30 LRP1flox/flox/LysM-Cre+ mice are characterized by the genetic inactivation of the LRP1 gene in cells expressing Lysozyme M, which include macrophages. Previous analysis of these mice has shown that more than 90% of these macrophages are deficient in LRP1, which has been confirmed for our colony.26,30 Housing and experiments were done as recommended by French regulations and the experimental guidelines of the European Community. Animal experiments were approved by the Animal Care and Use Committee of Inserm (license B-94-043-13).
Plasmids and hydrodynamic injection
The cDNA encoding a secretable receptor–associated protein (RAP) lacking the endoplasmic reticulum-retention sequence HNEL was kindly provided by Dr G. Bu (Mayo Clinic, Jacksonville, FL).31 The cDNA encoding neurophil-inhibiting factor (NIF)32 was assembled synthetically (GeneArt). Both cDNAs were modulated to add a C-terminal HPC4-tag and cloned into the pLIVE-expression plasmid (Mirus Bio), in which a mouse albumin promotor drives hepatocyte-specific expression.8,33 pLIVE-RAP, pLIVE-NIF, or empty pLIVE (each 100 μg) was applied to mice (20-25 g) with the use of the hydrodynamic injection method as described previously,8,33 resulting in at least 7 days of stable expression of both inhibitors. In all experiments, mice were used 4 days after hydrodynamic injection. Expression levels were quantified via dot-blot analysis, revealing plasma levels for RAP and NIF to be 1.5 μg/mL (range, 0.5-4 μg/mL) and 2 μg/mL (range, 0.5-8 μg/mL), respectively. Previous studies have shown that these levels are sufficient for a substantial inhibition of the LDL-receptor family and αMβ2, respectively.15,34,35
Proteins and protein assays
Plasma-derived VWF was purified from therapeutic VWF concentrates (Wilfactin, LFB Biomédicaments) via size-exclusion chromatography (110 U of VWF antigen/mg protein; FVIII, < 0.1 U/mg protein). Kogenate-FS (Bayer Healthcare) was used as source for recombinant FVIII. Purified endotoxin-free RAP was a kind gift of Dr S. Lacroix-Desmazes (Paris, France). Placenta-derived LRP1 was obtained from BioMac. Bovine serum albumin (BSA) was obtained from Sigma-Aldrich. FVIII, FIX, and FX activity were measured using 2-stage clotting assays. VWF antigen was quantified as described previously,5 except that monoclonal human anti-VWF antibodies were used as catching antibodies. Normal pooled plasma of 20 C57BL/6J mice was used as a reference and set at 1 U/mL. Results are expressed as units per milliliter relative to this standard.
Bleeding time
Mice were anesthetized with tribromoethanol (0.15 mL/10 g body weight), and 3 mm of the distal tail was cut with a scalpel. The tail was immersed immediately in saline buffer at 37°C. Bleeding time was measured from the moment of transection until the arrest of bleeding.
Clearance studies
Clearance of human VWF in macLRP1+ and macLRP1− mice was performed essentially as described previously.5 Clearance of human FVIII was determined by the injection of recombinant FVIII (150 U/kg) into the tail vein of macLRP1+ or macLRP1− mice. Blood samples were taken via retro-orbital puncture, and plasma was prepared to analyze residual FVIII levels using an in-house ELISA specific for human FVIII. Data over a 24-hour period were fitted with the use of Prism (Version 5 for Mac OSX; GraphPad Software), and the best fits were obtained with an equation describing a biexponential decay.5
Preparation of VWF-coated microspheres
Purified plasma-derived VWF and BSA were adsorbed onto fluorescent beads (Fluoresbrite-YG microspheres, 2.0 μm; Polysciences) according to the manufacturer's instructions. In brief, microspheres (250 μL containing 1.4 × 109 beads) were washed 3 times with 0.1M boric acid (pH 8.5) and subsequently incubated with 25, 50, or 200 μg of VWF or BSA for 16 hours under gentle swirling at room temperature. Microspheres were collected and resuspended in 0.1M boric acid (pH 8.5) containing 10 mg/mL BSA and incubated for 30 minutes at room temperature under gentle swirling, and this step was repeated another 2 times. Finally, coated microspheres were washed twice in 0.15M NaCl, 2.5mM CaCl2, 5% (vol/vol) glycerol, and 0.1M HEPES (pH 7.4) supplemented with 10 mg/mL BSA and stored at 4°C for 4 weeks maximum.
Static adhesion of VWF microspheres to LRP1
Microtiter wells were coated with LRP1, BSA, or anti-VWF antibody 539 (all at 0.5 μg/well) in a volume of 0.1 mL for 2 hours at 37°C in 0.1M NaHCO3 (pH 9.8). After washing wells 3 times with 0.15M NaCl, 2.5mM CaCl2, and 20mM Tris-HCl (pH 7.5; TBS), wells were blocked with TBS/0.5% (wt/vol) polyvinylpyrrolidone for 1 hour at 37°C. Subsequently, wells were incubated with VWF- or BSA-coated beads (1 × 104 beads/well) in a volume of 50 μL for 1 hour at room temperature. Wells were then washed 3 times with TBS/0.1% (vol/vol) Tween 20, and adhered microspheres were visualized via light microscopy.
Adhesion of VWF microspheres to LRP1 under flow
Adhesion of VWF- or BSA-coated beads to an LRP1-coated surface under flow was studied with Bioflux perfusion equipment (Fluxion Biosciences). Channels of Bioflux plates (48 wells) were coated with purified LRP1 or BSA (50 μg/mL, overnight at 4°C), and nonoccupied sites were blocked via incubation with polyvinylpyrrolidone (0.5%) for 30 minutes at room temperature. VWF- or BSA-coated microspheres (0.7 × 109 beads/mL; 50 μg/1.4 × 109 beads) were perfused at a shear of 2.5, 5, or 10 dyne/cm2. Alternatively, VWF- or BSA-coated microspheres (0.7 × 109 beads/mL; 25, 50, or 200 μg/1.4 × 109 beads) were perfused at a shear of 5 dyne/cm2. Specificity also was tested by perfusing VWF- or BSA-coated microspheres (0.7 × 109 beads/mL; 50 μg/1.4 × 109 beads) in the presence of RAP (20 μg/mL). Perfusion was followed via real-time microscopy. Snapshots of 5 different fields were taken after 2.5 minutes of perfusion within a period of 15 seconds. Adhesion was quantified by counting individual fluorescent microspheres using ImageJ 1.44 software (National Institutes of Health; http://rsbweb.nih.gov/ij/index.html).
Statistical analysis
Data are expressed as means (± SD), unless indicated otherwise. Multiple comparisons were performed using 1-way ANOVA. Statistical analysis of continuous parameters was performed with the Student unpaired t test, and Welch correction was applied when appropriate. In cases where VWF and FVIII levels were analyzed before and after treatment, we performed a paired t test analysis. P values less than .05 are considered statistically significant.
Results
Blocking LDL receptors results in increased VWF levels independently of FVIII
Previously, a combined increase in both FVIII and VWF was observed after genetic inactivation of hepatic LRP1.15 Because VWF has been reported not to interact with LRP1 itself and circulates in complex with FVIII, it cannot be excluded that this rise in VWF levels is subsequent to the rise in FVIII. To test this possibility, we determined VWF antigens levels in FVIII-deficient mice after hydrodynamic gene transfer of RAP, a protein that efficiently inhibits LRP1 and other members of the LDL-receptor family. Control FVIII-deficient mice treated with an empty pLIVE plasmid displayed similar VWF levels as untreated FVIII-deficient mice (FVIII-deficient + pLIVE-empty, 1.1 ± 0.1 U/mL [n = 4]; FVIII-deficient, 1.0 ± 0.3 U/mL [n = 10]; P = .45; Figure 1) 4 days after gene transfer, indicating that VWF levels were normal at this time point after the procedure. In contrast, expression of RAP in FVIII-deficient mice resulted in VWF levels that were increased almost 2-fold (1.9 ± 0.3 U/mL [n = 5]; P < .0001 compared with untreated FVIII-deficient mice; Figure 1). This demonstrates that the increase in VWF levels after inhibition of LDL receptors proceeds independently of FVIII.
Expression of RAP increases VWF levels in FVIII-deficient mice. Plasma samples were collected from untreated FVIII-deficient mice and FVIII-deficient mice subjected to hydrodynamic gene transfer with an empty pLIVE-expression plasmid (pLIVE-empty) or with pLIVE-RAP. VWF antigen levels are expressed in unit per milliliter relative to pooled normal mouse plasma. VWF antigen (Ag) levels are significantly increased in pLIVE-RAP–treated mice compared with untreated and pLIVE-empty–treated mice (1-way ANOVA, P < .0001). Data represent mean ± SD of 4 to 10 mice.
Expression of RAP increases VWF levels in FVIII-deficient mice. Plasma samples were collected from untreated FVIII-deficient mice and FVIII-deficient mice subjected to hydrodynamic gene transfer with an empty pLIVE-expression plasmid (pLIVE-empty) or with pLIVE-RAP. VWF antigen levels are expressed in unit per milliliter relative to pooled normal mouse plasma. VWF antigen (Ag) levels are significantly increased in pLIVE-RAP–treated mice compared with untreated and pLIVE-empty–treated mice (1-way ANOVA, P < .0001). Data represent mean ± SD of 4 to 10 mice.
Increased FVIII and VWF plasma levels in macLRP1− mice
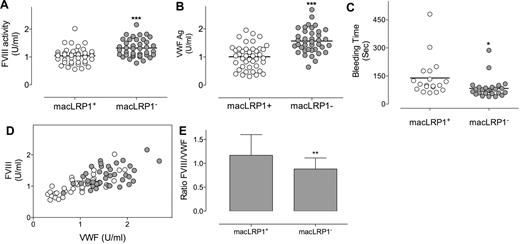
Given the dominant role of macrophages in the clearance of VWF,14 we chose to investigate the role of macrophage LRP1 in the catabolism of VWF. Mice were used that are homozygous for a loxP-flanked LRP1 gene and that express Cre-recombinase under the macrophage-specific promotor LysM (LRP1flox/flox/LysM-Cre+ mice, referred to as macLRP1− mice), with LRP1 being absent in more than 90% of macrophages in these macLRP1− mice.26,30 As control mice, LRP1flox/flox mice that do not express Cre-recombinase were used (referred to as macLRP1+ mice).27 MacLRP1+ and macLRP1− mice were similar with regard to plasma levels of coagulation factors IX and X (Table 1), both of which do not interact with LRP1. In contrast, FVIII levels were slightly but significantly higher in macLRP1− mice compared with control mice (1.3 ± 0.3 U/mL [n = 39] vs 1.0 ± 0.3 U/mL [n = 42]; P < .001; Table 1; Figure 2A). Interestingly, VWF levels also were modestly increased in macLRP1− mice compared with macLRP1+ mice (1.6 ± 0.4 U/mL vs 1.0 ± 0.4 U/mL; P < .001; Table 1; Figure 2B). We next tested whether increased levels of FVIII and VWF were associated with an increased hemostatic potential using a tail-clip bleeding assay. The average time to the arrest of bleeding was 2.3 ± 1.7 minutes in macLRP1+ mice (n = 19), and this value was significantly reduced to 1.4 ± 0.9 minutes in macLRP1− mice (n = 26; P = .039; Figure 2C). Altogether, these data indicate that the macrophage-specific deficiency of LRP1 results in elevated plasma levels of both FVIII and VWF in vivo that are associated with a slightly higher hemostatic potential.
FVIII and VWF levels in macLRP1+ and macLRP1− mice. Plasma samples were collected from age- and sex-matched macLRP1+ mice (n = 42; open circles) and macLRP1− mice (n = 39; closed circles) and analyzed for FVIII (A) and VWF (B). Individual FVIII levels also were plotted versus individual VWF levels (D). For each mouse, the ratio FVIII over VWF was calculated. The average ratio (± SD) for macLRP1+ and macLRP1− is provided in panel E. In another group of macLRP1+ (n = 19; open circles) and macLRP1− mice (n = 26; closed circles), the time to arrest of bleeding in a tail-clip bleeding model (see “Bleeding time”; ***P < .0001, **P < .001, *P < .05) was determined (C).
FVIII and VWF levels in macLRP1+ and macLRP1− mice. Plasma samples were collected from age- and sex-matched macLRP1+ mice (n = 42; open circles) and macLRP1− mice (n = 39; closed circles) and analyzed for FVIII (A) and VWF (B). Individual FVIII levels also were plotted versus individual VWF levels (D). For each mouse, the ratio FVIII over VWF was calculated. The average ratio (± SD) for macLRP1+ and macLRP1− is provided in panel E. In another group of macLRP1+ (n = 19; open circles) and macLRP1− mice (n = 26; closed circles), the time to arrest of bleeding in a tail-clip bleeding model (see “Bleeding time”; ***P < .0001, **P < .001, *P < .05) was determined (C).
Relation between FVIII and VWF levels in macLRP1+ and macLRP1− mice
VWF is a major determinant of FVIII plasma levels.11–13 To assess the effect of LRP1 on the correlation between plasma levels of both proteins, FVIII levels were plotted versus VWF levels (Figure 2D). For both mouse strains, a clear correlation between FVIII and VWF levels was observed, in that low FVIII levels were associated with low VWF levels and high FVIII levels with high VWF levels. Pearson rank correlations were 0.77 (P < .0001) and 0.50 (P = .0011) for macLRP1+ and macLRP1− mice, respectively. Interestingly, the ratio FVIII over VWF was slightly but significantly reduced in macLRP1− mice compared with macLRP1+ mice (ratio, 0.88 ± 0.23 and 1.17 ± 0.43, respectively; P = .001; Figure 2E). This suggests that VWF levels are increased to a larger extent than FVIII levels after the inactivation of LRP1 in macrophages.
macLRP1 dominates the effect on modulation of VWF plasma levels
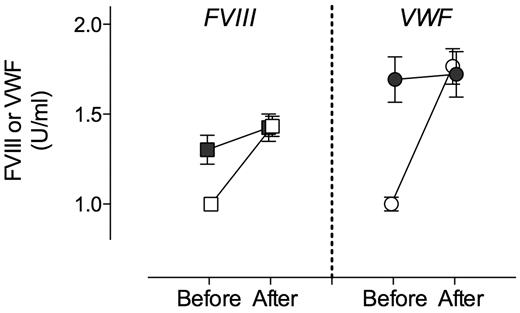
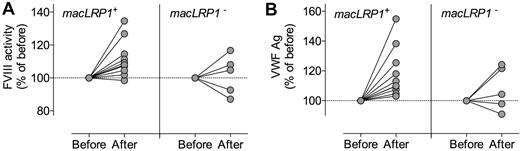
It has been shown that besides LRP1, other LDL receptors contribute to the catabolism of FVIII. In addition, LRP1 expression includes other cell types, such as hepatocytes, endothelial cells, and stellate cells, all of which are present in liver. To explore the potential contribution of other LDL-like receptors, other cell types, or both to the modulation of VWF plasma levels, macLRP1− mice were subjected to hydrodynamic gene transfer with RAP. As control, macLRP1+ mice were treated to express RAP. As expected, expression of RAP in macLRP1+ mice resulted in a marked increase of FVIII (from 1.0 ± 0.1 U/mL to 1.4 ± 0.2 U/mL; P < .0001; Figure 3). An increase in FVIII also was found in every individual macLRP1− mouse after expression of RAP. Of note, the rise in FVIII (mean rise, 0.10 U/mL; 95% confidence interval [CI], 0.04-0.17 U/mL; P = .004) was less extensive than observed in macLRP1+ mice. Nevertheless, these data conform that LRP1 expressed in other cells and other members of the LDL-receptor family contribute to the catabolism of FVIII in addition to macLRP1. As for VWF, we detected a significant increase in plasma levels after expression of RAP in macLRP1+ mice (from 1.0 ± 0.1 U/mL to 1.8 ± 0.3 U/mL; P < .0001; Figure 3). In contrast, no significant effect on VWF levels was observed when RAP was expressed in macLRP1− mice (mean change, 0.11 U/mL; 95% CI, −0.07% to 0.29%; P > .05). Apparently, macLRP1 plays a more important role in comparison with LRP1 in other cells or other lipoprotein receptors in the regulation of VWF plasma levels.
Effect of RAP expression on FVIII and VWF levels in macLRP1+ and macLRP1− mice. Plasma samples of MacLRP1+ mice (open symbols; n = 10) and macLRP1− (closed symbols; n = 10) were taken 5 days before and 4 days after hydrodynamic gene transfer with pLIVE-RAP. Samples were analyzed for FVIII activity (squares) and VWF antigen (circles) levels. Data represent mean ± SD. FVIII and VWF levels before and after treatment were assessed for statistical significance using a paired t test. A statistically significant increase in FVIII activity was found in macLRP+ and macLRP1− mice, whereas for VWF a statistically significant increase was found in macLRP+ but not macLRP− mice.
Effect of RAP expression on FVIII and VWF levels in macLRP1+ and macLRP1− mice. Plasma samples of MacLRP1+ mice (open symbols; n = 10) and macLRP1− (closed symbols; n = 10) were taken 5 days before and 4 days after hydrodynamic gene transfer with pLIVE-RAP. Samples were analyzed for FVIII activity (squares) and VWF antigen (circles) levels. Data represent mean ± SD. FVIII and VWF levels before and after treatment were assessed for statistical significance using a paired t test. A statistically significant increase in FVIII activity was found in macLRP+ and macLRP1− mice, whereas for VWF a statistically significant increase was found in macLRP+ but not macLRP− mice.
Clearance of FVIII and VWF is modulated after inactivation of macLRP1
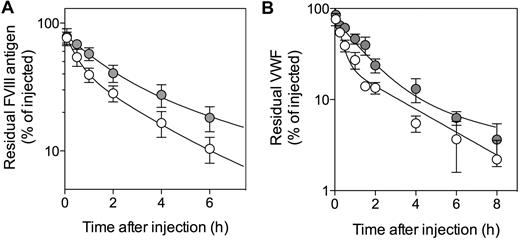
One possibility that explains increased plasma levels of VWF is that it is cleared less rapidly. We therefore examined VWF clearance in both mouse strains. We also tested clearance of FVIII, the survival of which is VWF-dependent. Injection of human FVIII results in complex formation of FVIII with mouse VWF.36 As shown in Figure 4A, FVIII was removed from the circulation less rapidly in macLRP1− mice compared with macLRP1+ mice. The mean residence time (MRT) was calculated to be 3.1 ± 0.7 and 5.5 ± 1.2 hours, respectively (P = .041). With regard to VWF, a similar prolongation was detected after the injection of highly purified human VWF: MRT = 2.8 ± 0.6 and 6.0 ± 0.6 hours (P = .0028) for macLRP1+ and macLRP1− mice, respectively (Figure 4B). These results strongly indicate that the absence of LRP1 in macrophages results in a reduced clearance of VWF.
Plasma clearance of FVIII and VWF in macLRP1+ and macLRP1− mice. MacLRP1+ mice (open circles) and macLRP1− mice (closed circles) were given recombinant FVIII (Kogenate; 150 U/kg; A) or pd-VWF (0.25 mg/kg; B). All reagents were given intravenously in the tail vein. Plasma samples were collected at indicated time points and analyzed for FVIII or VWF antigen, respectively. Data represent percentage of residual antigen compared with the amount injected. For each time point, the mean ± SD from 3 to 9 individual mice is shown. For calculation of MRT, data over a 24-hour period were used. The drawn lines were obtained from fitting the data to an equation describing a biexponential decay.
Plasma clearance of FVIII and VWF in macLRP1+ and macLRP1− mice. MacLRP1+ mice (open circles) and macLRP1− mice (closed circles) were given recombinant FVIII (Kogenate; 150 U/kg; A) or pd-VWF (0.25 mg/kg; B). All reagents were given intravenously in the tail vein. Plasma samples were collected at indicated time points and analyzed for FVIII or VWF antigen, respectively. Data represent percentage of residual antigen compared with the amount injected. For each time point, the mean ± SD from 3 to 9 individual mice is shown. For calculation of MRT, data over a 24-hour period were used. The drawn lines were obtained from fitting the data to an equation describing a biexponential decay.
VWF interferes with FVIII binding to LRP1 in SPR analysis
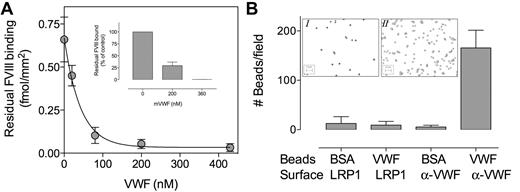
The observation that the lack of LRP1 modulates VWF clearance is surprising, given that it has been reported that VWF does not interact with LRP1.16,17 Indeed, in control experiments we could not detect binding of VWF to LRP1, when using surface plasmon resonance (SPR) analysis (BIAcore2000 analyzer; BIAcore) or immunosorbent assays. In contrast, we could confirm that FVIII binds efficiently to LRP1 in a dose-dependent manner using SPR analysis, with half-maximal binding being achieved at 51 ± 10nM (data not shown). Again, binding of FVIII (40nM) to LRP1 was progressively inhibited in the presence of increasing concentrations of VWF (Ki,app, 13 ± 2nM; Figure 5A). Similarly, binding of FVIII to LRP1 also was inhibited by recombinant murine VWF in an immunosorbent assay (Figure 5A inset).
Lack of VWF binding to LRP1during SPR analysis and under static adhesion. (A) LRP1 immobilized at a CM5-sensorchip (12 fmol/mm2) was incubated with FVIII (40nM) in the presence of various concentrations of VWF (0-430nM) in 0.15M NaCl, 2mM CaCl2, 0.005% Tween 20, and 20mM HEPES (pH 7.4) at a flow of 20 μL/min at 25°C. Residual FVIII binding at equilibrium (femtomoles per square millimeter) versus VWF concentration (nanomolar) is plotted. Inset, LRP1 (0.5 μg/well) was immobilized in microtiter wells and incubated with recombinant FVIII (2nM) in the absence or presence of recombinant murine VWF (0-360nM). Residual FVIII bound was detected using monoclonal anti-FVIII antibody D4H1. Percentage of residual FVIII bound compared with binding in the absence of VWF is plotted. (B) LRP1 and anti-VWF antibody 539 (both 0.5 μg/well) were immobilized in microtiter wells and subsequently incubated with VWF- or BSA-coated microspheres (Fluoresbrite-YG, 2 μm) for 1 hour at room temperature. Wells were washed, and adhered microspheres were quantified via light microscopy. Data represent the number of microspheres per field ± SD. Twelve fields (125 × 95 μm) were examined, with a maximum of 4 fields/well. Images display a representative field of VWF-coated beads incubated with LRP1 (inset I) or anti-VWF antibody 539 (inset II).
Lack of VWF binding to LRP1during SPR analysis and under static adhesion. (A) LRP1 immobilized at a CM5-sensorchip (12 fmol/mm2) was incubated with FVIII (40nM) in the presence of various concentrations of VWF (0-430nM) in 0.15M NaCl, 2mM CaCl2, 0.005% Tween 20, and 20mM HEPES (pH 7.4) at a flow of 20 μL/min at 25°C. Residual FVIII binding at equilibrium (femtomoles per square millimeter) versus VWF concentration (nanomolar) is plotted. Inset, LRP1 (0.5 μg/well) was immobilized in microtiter wells and incubated with recombinant FVIII (2nM) in the absence or presence of recombinant murine VWF (0-360nM). Residual FVIII bound was detected using monoclonal anti-FVIII antibody D4H1. Percentage of residual FVIII bound compared with binding in the absence of VWF is plotted. (B) LRP1 and anti-VWF antibody 539 (both 0.5 μg/well) were immobilized in microtiter wells and subsequently incubated with VWF- or BSA-coated microspheres (Fluoresbrite-YG, 2 μm) for 1 hour at room temperature. Wells were washed, and adhered microspheres were quantified via light microscopy. Data represent the number of microspheres per field ± SD. Twelve fields (125 × 95 μm) were examined, with a maximum of 4 fields/well. Images display a representative field of VWF-coated beads incubated with LRP1 (inset I) or anti-VWF antibody 539 (inset II).
Shear stress–induced adhesion of VWF to immobilized LRP1
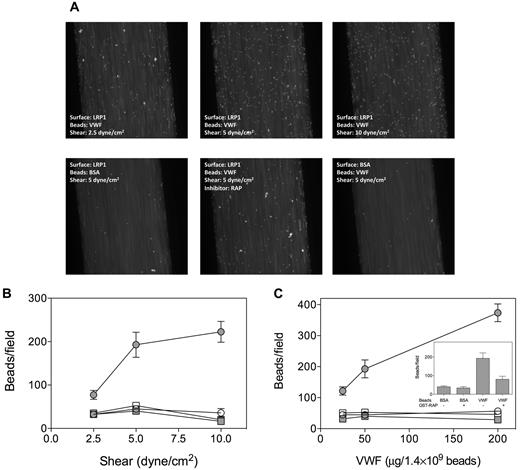
Because some VWF-mediated interactions rely on shear-induced conformations within the VWF molecule, we tested the adhesion of VWF to LRP1 under flow conditions. To facilitate the detection of VWF adhering to LRP1, VWF was adsorbed onto fluorescent microspheres (Fluoresbrite-YG, 2 μm). First, adhesion of VWF- and BSA-coated microspheres to immobilized LRP1 or the anti-VWF antibody 539 was examined under static conditions. Compared with BSA-coated beads, VWF-coated beads efficiently adhered to anti-VWF antibody 539 (Figure 5B). In contrast, little adhesion to LRP1 was observed for both BSA- and VWF-coated beads, confirming that VWF does not bind to LRP1 under static conditions. We then perfused VWF- or BSA-coated microspheres over LRP1- or BSA-coated channels at shear forces of 2.5, 5, or 10 dyne/cm2. Similar background adhesion was observed for VWF-coated beads and BSA-coated beads on a BSA-coated surface and BSA-coated beads on an LRP1-coated surface in a manner that was shear stress–independent (Figure 6). In contrast, VWF-coated beads displayed shear stress-dependent adhesion to LRP1, with the number of adhered beads per field increasing from 77 to 223 (p-trend = .0028; Figure 6B). Such shear stress–induced increase in adhesion was not observed when RAP-coated beads were perfused over LRP1 (data not shown). We tested the specificity of the adhesion by perfusing beads coated with different amounts of VWF or BSA. A dose-dependent increase in adhesion was observed for VWF-coated beads that were perfused over LRP1 at 5 dyne/cm2 (number of beads increasing from 120 to 375/field; p-trend < .0001) but not for controls (Figure 6C). In addition, the presence of RAP (20 μg/mL) significantly inhibited adhesion of VWF-coated beads to LRP1 (193 ± 100 vs 80 ± 37 beads/field; P = .03; Figure 6C inset). These perfusion assays strongly suggest the existence of shear stress–dependent interactions between VWF and LRP1.
Adhesion of VWF-coated beads to immobilized LRP1 under flow. Fluoresbrite-YG microspheres were coated with BSA or VWF (25, 50, or 200 μg/1.4 × 109 beads) and perfused over BSA- or LRP1-coated channels (shear force: 2.5, 5, or 10 dyne/cm2) using Bioflux microfluidic equipment. (A) Snapshots of VWF- or BSA-coated microspheres being perfused (t = 2 minutes) over LRP1- or BSA-coated channels. (B) Quantification of adhesion of beads coated with 50 μg/1.4 × 109 beads at shear forces of 2.5, 5, or 10 dyne/cm2. (C) Quantification of adhesion of beads coated with 25, 50, or 200 μg/1.4 × 109 beads at 5 dyne/cm2. Inset, Quantification of adhesion of beads coated with 50 μg/1.4 × 109 beads at 5 dyne/cm2 in the absence or presence of RAP (20 μg/mL). Adhesion was quantified by counting the number of adhered microspheres in 5 microscopic fields taken after 2.5 minutes of perfusion within a period of 15 seconds using ImageJ 1.44 software. Data represent the mean ± SEM of 3 to 6 perfusions. Closed circles indicate VWF beads and LRP1 coating; open circles, VWF beads and BSA coating; closed squares, BSA beads and LRP1 coating; and open squares, BSA beads and BSA coating.
Adhesion of VWF-coated beads to immobilized LRP1 under flow. Fluoresbrite-YG microspheres were coated with BSA or VWF (25, 50, or 200 μg/1.4 × 109 beads) and perfused over BSA- or LRP1-coated channels (shear force: 2.5, 5, or 10 dyne/cm2) using Bioflux microfluidic equipment. (A) Snapshots of VWF- or BSA-coated microspheres being perfused (t = 2 minutes) over LRP1- or BSA-coated channels. (B) Quantification of adhesion of beads coated with 50 μg/1.4 × 109 beads at shear forces of 2.5, 5, or 10 dyne/cm2. (C) Quantification of adhesion of beads coated with 25, 50, or 200 μg/1.4 × 109 beads at 5 dyne/cm2. Inset, Quantification of adhesion of beads coated with 50 μg/1.4 × 109 beads at 5 dyne/cm2 in the absence or presence of RAP (20 μg/mL). Adhesion was quantified by counting the number of adhered microspheres in 5 microscopic fields taken after 2.5 minutes of perfusion within a period of 15 seconds using ImageJ 1.44 software. Data represent the mean ± SEM of 3 to 6 perfusions. Closed circles indicate VWF beads and LRP1 coating; open circles, VWF beads and BSA coating; closed squares, BSA beads and LRP1 coating; and open squares, BSA beads and BSA coating.
Inhibition of β2-integrins in vivo results in increased VWF levels
Although LRP1 seems able to interact with VWF directly under flow conditions, we also considered our previous findings that (1) VWF is able to interact with β2-integrins, and (2) LRP1 is needed for optimal β2-integrin function.22,25 As such, the absence of LRP1 could potentially modulate binding and uptake of VWF by β2-integrins expressed by macrophages. To test this hypothesis, mice were subjected to hydrodynamic gene transfer with NIF, an inhibitor of αMβ2-integrin.37 Control mice were given an empty expression plasmid. Efficiency of αMβ2-integrin inhibition was tested by measuring fibrinogen levels, a protein that is bound and endocytosed via this integrin.38 Fibrinogen levels were 0.8 ± 0.1 mg/mL (n = 5) in pLIVE-empty–treated mice and 1.0 ± 0.1 mg/mL in pLIVE-NIF–treated mice (n = 5; P = .0157). These increased fibrinogen levels suggest that circulating NIF efficiently inhibits αMβ2. A modest but statistically significant rise in both FVIII and VWF was observed after the expression of the αMβ2-integrin inhibitor NIF in macLRP+ mice: 0.13 U/mL (95% CI, 0.04-0.21 U/mL; P = .008) and 0.18 U/mL (95% CI, 0.08-0.29 U/mL; P = .004) for FVIII and VWF, respectively (Figure 7). Interestingly, no further increase in FVIII or VWF levels was observed when NIF was expressed in macLRP1− mice: 0.02 U/mL (95% CI, −0.13 to 0.17 U/mL; P > .05) and 0.08 U/mL (95% CI, −0.11 to 0.27 U/mL; P > .05) for FVIII and VWF, respectively (Figure 7). Apparently, αMβ2-integrins are able to contribute to the regulation of VWF levels to a minor extent, but they do so in an LRP1-dependent manner.
Effect of NIF expression on VWF levels. Blood was collected from mice 5 days before and 4 days after hydrodynamic gene transfer with pLIVE-NIF in macLRP1+ mice (n = 10) or macLRP1− mice (n = 5). Plasma was prepared and analyzed for FVIII activity (A) and VWF antigen (B). Data are presented as percentage of pretreatment levels. FVIII and VWF levels before and after treatment were assessed for statistical significance using a paired t test. A statistically significant increase in FVIII and VWF levels was found in macLRP+ mice but not in macLRP1− mice.
Effect of NIF expression on VWF levels. Blood was collected from mice 5 days before and 4 days after hydrodynamic gene transfer with pLIVE-NIF in macLRP1+ mice (n = 10) or macLRP1− mice (n = 5). Plasma was prepared and analyzed for FVIII activity (A) and VWF antigen (B). Data are presented as percentage of pretreatment levels. FVIII and VWF levels before and after treatment were assessed for statistical significance using a paired t test. A statistically significant increase in FVIII and VWF levels was found in macLRP+ mice but not in macLRP1− mice.
Discussion
The fact that VWF and FVIII circulate in complex implies that clearance receptors recognizing either protein may contribute to the clearance of the complex. One such example includes LRP1. Previously, LRP1 has been identified as a receptor for FVIII.15–17 In contrast, in vitro studies (including our own) showed that LRP1 did not interact with VWF and that VWF actually inhibits binding of FVIII to LRP1.16,17 Yet, in vivo analysis revealed that genetic inactivation of the LRP1 gene not only results in increased plasma levels of FVIII but also in increased plasma levels of VWF.15 Moreover, the LRP1 polymorphism D2080N is associated with VWF plasma levels in the normal population.39,40 An explanation for this apparent contradiction between the in vitro and in vivo observations could be that FVIII within the FVIII-VWF complex is recognized in an LRP1-dependent manner in vivo but not in vitro. If this were true, then blocking LRP1 in vivo would result in an indirect FVIII-mediated increase in VWF levels. We tested this hypothesis by blocking LRP1 with RAP in FVIII-deficient mice. However, we observed the opposite in that VWF levels were almost doubled in FVIII-deficient mice in the presence of the LRP1 inhibitor RAP (Figure 1), implying that LRP1 directly regulates VWF plasma levels rather than in an FVIII-dependent manner.
To further investigate the link between LRP1 and VWF, we used an experimental mouse model with a specific inactivation of the floxed LRP1 gene in LysM-expressing cells that include macrophages, cells that dominate in the clearance of VWF.14 Indeed, both FVIII and VWF levels were increased in these mice, whereas levels of factors IX and X were unaffected (Table 1). The increase in FVIII levels was less pronounced in macLRP1− mice compared with mice in which LRP1 was inactivated in the complete liver (thus including, eg, hepatocytes, macrophages), where FVIII levels were increased up to 3.4 U/mL.15 This indicates that other cells than macrophages and other members of the LDL-receptor family than macLRP1 can contribute to the regulation of its plasma levels. In line with this finding, we observed that FVIII levels were further increased in macLRP1− mice after the expression of RAP (Figure 3). Surprisingly, no further increase in VWF levels was observed in macLRP1− mice after the expression of RAP (Figure 3), indicating that regulation of VWF levels seems to be mediated by macrophage-expressed LRP1. This is compatible with our previous findings that VWF is selectively targeted to macrophage cells rather than other cells within the liver.14 It should be noted that an increase in VWF levels was observed in the study by Bovenschen et al, after adenoviral-mediated expression of RAP.15 Possibly, the RAP levels achieved in our system are too low to block all members of the LDL-receptor family. However, similar plasma levels of RAP (0.7-2.4 μg/mL) have been shown previously to result in a substantial inhibition of these lipoprotein receptors.15,34 Alternatively, it has been reported previously that the use of adenovirus results in rapid Kupffer cell death,41 that is, the cells that mediate clearance of VWF.14 Indeed, application of adenoviral vectors is associated with a concomitant rise of VWF42 that may obscure the net effect of RAP. In addition, we have shown previously that VWF also may be cleared via macrophages outside the liver.5 The possibility exists that the overexpression of RAP in the Bovenschen et al15 study contributes to the inhibition of LRP1 in these extra-hepatic macrophages, thereby blocking extra-hepatic clearance of VWF.
MacLRP1 may modulate VWF levels by influencing biosynthesis and secretion, clearance, or a combination. The potential effect of macLRP1 on VWF biosynthesis and secretion seems unlikely, given that VWF is not produced in macrophages but in endothelial cells and megakaryocytes. However, it cannot be excluded that because of the absence of macLRP1, an excess of certain LRP1 ligands in the circulation results in a chronic stimulation of endothelial cells and subsequent release of VWF. Such ligands will not include cholesterol or triglycerides, because their levels have been found to be normal in macLRP1− mice.26 In the current study, we have focused on the possibility that macLRP1 modulates clearance of VWF. Indeed, we did see a longer survival of both FVIII and VWF in the absence of macLRP1 (Figure 3). Importantly, the 2-fold increase in MRT is modest, suggesting that apart from LRP1-dependent mechanisms also other pathways may play a role in the clearance of VWF and its complex with FVIII (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
One intriguing aspect of the study was how LRP1 modulates clearance of VWF. As mentioned, previous studies by us and others have reported that VWF is unable to interact with LRP1. Here, we confirmed this lack of interaction in SPR-based experiments and in static adhesion experiments using VWF-coated microspheres (Figure 5). We explored 2 possibilities that could explain this apparent discrepancy. First, it is well known that the conformation of VWF relies on the shear stress to which VWF is exposed, a phenomenon that has been visualized using different experimental approaches.43,44 This conformational change is needed to convert VWF into an active, platelet-binding conformation.45 To our surprise, we observed that VWF-coated microspheres adhered efficiently to LRP1-coated channels under conditions of flow, whereas these beads did not adhere to LRP1 under static conditions (Figures 5 and 6). Adhesion was not only dose-dependent but also shear stress–dependent, with the amount of adhered beads increasing with higher shear. It should be noted that no binding of VWF to immobilized LRP1 is detected when using BIAcore 2000 equipment (eg, Figure 5A), despite that VWF is perfused over LRP1. It is possible that under the applied conditions, the achieved shear rates are insufficient to induce LRP1 binding. Indeed, a shear force of 1.6 dyne/cm2 can be calculated when applying a flow rate of 20 μL/min to the dimensions of the perfusion area in the BIAcore 2000 analyzer. A second issue that could influence VWF binding to LRP1 in the BIAcore 2000 system is that LRP1 is immobilized via an amine-coupling procedure that may result in the masking of VWF binding sites. Nevertheless, the requirement of shear for VWF-LRP1 interactions to happen is unexpected but seems to explain the discrepancy between previous in vitro and in vivo findings with regard to the effect of LRP1 deficiency on VWF levels. Interestingly, our findings are in full agreement with a recent preliminary report by Castro et al, who suggested that the uptake of the FVIII-VWF complex is more efficient under conditions of shear and that this process can be inhibited using RAP.46 One may further wonder whether the shear stress dependency for the VWF-LRP1 interaction fits with the size-independent clearance of VWF that we have reported previously.5 It is important to realize that not only larger multimers but also small-sized multimers can be present in an active platelet-binding conformation.47 This also suggests that small-sized multimers seem to be responsive to shear stress-induced conformational changes, allowing them to interact with LRP1.
Another link between LRP1 and VWF that we explored was related to β2-integrins. We have shown previously that LRP1 is able to form a heterologous complex with leukocyte-specific β2-integrins at the cell surface.22 Deletion of LRP1 leaves β2-integrin surface expression unaffected, but it impairs redistribution of the integrins to the cholesterol-enriched membrane fractions, resulting in reduced adhesion capacities.22 Because 1 of these β2-integrins, αMβ2, is abundantly expressed in macrophages, it seems possible that both αMβ2 and LRP1 associate in these cells. We therefore challenged the hypothesis that β2-integrins may contribute to the uptake of VWF, a process that would depend on the presence of macLRP1. This was tested by inhibiting β2-integrins in control mice via the expression of NIF that efficiently blocks αMβ2-integrin.32 Expression of NIF was associated with a small but statistically significant increase in FVIII and VWF levels (0.13 and 0.18 U/mL, respectively) in macLRP+ but not in macLRP− mice (Figure 7). This small increase points to but a minor role of β2-integrins in the regulation of VWF and FVIII plasma levels. However, the absence of such rise in FVIII/VWF levels in macLRP− mice further strengthens the previous findings that LRP1 and β2-integrins form a functional complex.22–24 Whether disruption of β2-integrin–LRP1 complexes reduces binding of VWF to β2-integrins or to LRP1 remains to be determined.
In conclusion, we have demonstrated that macrophage LRP1 modulates clearance of VWF and its complex with FVIII. LRP1 may do so via direct shear stress–induced interactions with VWF (while blood containing VWF is flowing along LRP1-expressing macrophages), or as part of a functional LRP1–β2-integrin complex. The notion that the lack of LRP1 in macrophages has a modest effect on VWF clearance points to the existence of alternative pathways (supplemental Figure 1). These may include the asialoglycoprotein receptor,48 CLEC4M,49 or members of the Siglec family.50
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study was supported by Agence Nationale de la Recherche (ANR) grants ANR-08-CEXC-018-01 and ANR-08-EBIO-026-01.
Authorship
Contribution: G.R., J.N.P., C.C., S.O., A.-M.N., N.S.-L., P.L., and P.J.L. performed experiments and analyzed data; B.J.v.V. provided LRP1-deficient mice; O.D.C., C.V.D., and P.J.L. designed research; G.R. and P.J.L. wrote manuscript; and all authors contributed to the editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter J. Lenting, Inserm U770, 80 rue du Général Leclerc, 94276 Le Kremlin-Bicêtre, France; e-mail: peter.lenting@inserm.fr.