Abstract
Multiple diseases, hematologic and nonhematologic, result from defects in the early secretory pathway. Congenital dyserythropoietic anemia type II (CDAII) and combined deficiency of coagulation factors V and VIII (F5F8D) are the 2 known hematologic diseases that result from defects in the endoplasmic reticulum (ER)–to–Golgi transport system. CDAII is caused by mutations in the SEC23B gene, which encodes a core component of the coat protein complex II (COPII). F5F8D results from mutations in either LMAN1 (lectin mannose-binding protein 1) or MCFD2 (multiple coagulation factor deficiency protein 2), which encode the ER cargo receptor complex LMAN1-MCFD2. These diseases and their molecular pathogenesis are the focus of this review.
Introduction
Multiple human diseases resulting from defects in the endoplasmic reticulum (ER)–to–Golgi transport have been reported over the past 14 years (Table 1). Anderson disease or chylomicron retention disorder (CMRD),1 a disease characterized by malabsorption of lipids from the diet and accumulation of chylomicrons in the enterocytes, results from mutations in SAR1B, a component of coat protein complex II (COPII)–coated vesicles that bud from the surface of the ER and transport cargo proteins to the Golgi apparatus. Patients with CMRD exhibit failure to thrive in infancy, absence of circulating chylomicrons, low levels of plasma cholesterol, and deficiency of fat-soluble vitamins. Mutations in SEC23A, another component of the COPII coat, result in cranio-lenticulo-sutural-dysplasia (CLSD),2 an autosomal recessive syndrome characterized by sutural cataracts, late closure of the cranial fontanelles, skeletal abnormalities, and dysmorphic facial features. Only 2 CLSD pedigrees have been reported, each with a different missense mutation.2,3
Defects in the ER export machinery have been reported to cause 2 hematologic diseases, combined deficiency of coagulation factors V (FV) and VIII (FVIII) (F5F8D) and congenital dyserythropoietic anemia type II (CDAII). F5F8D is characterized by a decrease in FV and FVIII levels to ∼ 10% of normal with generally mild bleeding manifestations. This disorder results from mutations in either LMAN14 (lectin mannose-binding protein 1) or MCFD25 (multiple coagulation factor deficiency protein 2), 2 genes that encode the components of a specific ER cargo receptor for FV and FVIII. CDAII results from mutation in SEC23B, a paralog of SEC23A in mammals. CDAII is characterized by mild to moderate anemia, bi- or multinucleated erythroblasts, aberrant glycosylation of erythrocyte band 3, and red blood cell (RBC) lysis in some (but not all) acidified normal sera.
Although no human diseases resulting from defects in other components of the early secretory pathway have been reported to date, animal models of defects in Sec24B, Sec24C, and Sec24D, have been described6-8 (Table 1), again reflecting a range of manifestations, from no apparent phenotype (zebrafish Sec24C),8 to a unique neural tube developmental defect (murine Sec24B)7,12 to characteristic skeletal malformations (zebrafish Sec24D).8
A general overview of the Mendelian disorders resulting from defects in the membrane trafficking machinery was recently published.13 In this review, we focus on the 2 hematologic diseases resulting from defects in the early secretory pathway, F5F8D and CDAII, and their molecular pathogenesis.
ER-to-Golgi transport
Approximately one-third of the proteins encoded by the mammalian genome are destined for the secretory pathway.14 The ER and Golgi apparatus are not only the initial stations of the secretory pathway, but also the common organelles through which all secreted proteins navigate while advancing toward their final destinations.
In the ER, proteins are folded and sorted. Some proteins stay in the ER as resident ER proteins, while the majority are transported to the Golgi apparatus for further posttranslational modification before reaching their final destinations—lysosomes, endosomes, plasma membrane, or the extracellular space. The proteins that are to be transported out of the ER are routed to ER exit sites where they are packaged into COPII-coated vesicles.
COPII-coated vesicles
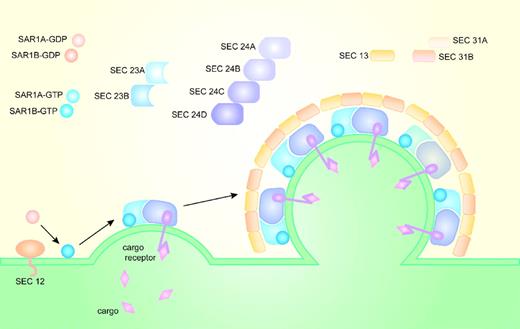
COPII coat composition and assembly has been extensively studied and well characterized in Saccharomyces cerevisiae.15-17 The core components of the COPII coat form 2 layers: an inner layer composed of the monomeric GTP-binding protein Sar1p and the heterodimeric complex Sec23p-Sec24p, and an outer layer formed by the heterotetrameric complex Sec13p-Sec31p. In addition to the core components of the COPII coat, Sec12p and Sec16p are important for in vivo vesicle formation.
COPII coat assembly (Figure 1) begins when the transmembrane guanine nucleotide exchange factor (GEF)—Sec12p—converts the cytosolic GDP-bound Sar1p (Sar1p-GDP) to GTP-bound Sar1p (Sar1p-GTP) and recruits it to the cytoplasmic ER membrane.18,19 ER membrane-bound Sar1p-GTP, through direct binding to sec23p, recruits the Sec23p-Sec24p heterodimer from the cytoplasm. The Sar1p-Sec23p-Sec24p complex assembled on the cytoplasmic surface of the ER membrane binds ER transmembrane protein cargos primarily through interactions between cargo and Sec24.20-23 Soluble cargo confined to the ER lumen are recruited to the nascent COPII vesicle through interaction with transmembrane cargo receptors. After cargo selection, the Sar1p-Sec23p-Sec24p prebudding complex recruits the heterotetramer proteins Sec13p-Sec31p from the cytosol to form the outer layer of the COPII coat, which facilitates budding of the vesicle from the surface of the ER membrane.24-27 Sec16p may help regulate COPII vesicle formation, and facilitate budding of the COPII vesicles.28
Formation of the COPII vesicle on the surface of the endoplasmic reticulum. In yeasts, Sec12p recruits Sar1p to the ER membrane and converts Sar1p-GDP into Sar1p-GTP. Sar1p-GTP, through direct binding to Sec23p, recruits Sec23p-Sec24p heterodimers from the cytoplasm to the ER membrane. Sar1p, Sec23p, and Sec24p form the inner layer of the COPII coat and the prebudding complex that recruits Sec13p-Sec31p heterotetramers. Sec13p-Sec31p form the outer layer of the COPII coat and facilitate budding of the COPII-coated vesicles. Cargo selection occurs primarily through direct or indirect interaction with Sec24. In mammals, there are 2 paralogs for Sar1, 2 for Sec23, 4 for Sec24, and 2 for Sec31.
Formation of the COPII vesicle on the surface of the endoplasmic reticulum. In yeasts, Sec12p recruits Sar1p to the ER membrane and converts Sar1p-GDP into Sar1p-GTP. Sar1p-GTP, through direct binding to Sec23p, recruits Sec23p-Sec24p heterodimers from the cytoplasm to the ER membrane. Sar1p, Sec23p, and Sec24p form the inner layer of the COPII coat and the prebudding complex that recruits Sec13p-Sec31p heterotetramers. Sec13p-Sec31p form the outer layer of the COPII coat and facilitate budding of the COPII-coated vesicles. Cargo selection occurs primarily through direct or indirect interaction with Sec24. In mammals, there are 2 paralogs for Sar1, 2 for Sec23, 4 for Sec24, and 2 for Sec31.
Shortly after a COPII vesicle buds from the ER membrane and as a feedback mechanism for its formation, Sec23p functions as a Sar1p GTPase-activating protein,29 thus resulting in uncoating of the COPII vesicle. The GTP hydrolysis activity of Sec23p is enhanced by Sec13p-Sec31p.30 After COPII uncoating, the vesicles fuse together forming the ER-Golgi intermediate compartment (ERGIC). Anterograde transport continues along microtubules to the Golgi apparatus.31,32 Retrograde transport from the Golgi and ERGIC to the ER is mediated via COPI vesicles.
COPII coat proteins in higher eukaryotes
The COPII proteins are evolutionarily conserved; orthologs of the core components of the yeast COPII coat exist in mammals and other higher eukaryotes. However, compared with the yeast genome where only SEC24 has identifiable paralogs (LST1 and ISS1),22 the mammalian genome contains multiple paralogs for each component of the COPII coat, with the exception of SEC13.33 There are 2 paralogs for SAR1 (SAR1A and SAR1B), 2 for SEC23 (SEC23A and SEC23B), 4 for SEC24 (SEC24A, SEC24B, SEC24C, and SEC24D), and 2 for SEC31 (SEC31A and SEC31B; Figure 1). The diverse paralogs for the COPII components in mammals could potentially contribute to a wide variety of COPII coats, potentially facilitating selective cargo transport in a tissue-specific manner. Alternative splicing34 could contribute further to the diversity of the COPII vesicle and cargo selection.
Cargo-mediated transport
Although ER transmembrane proteins can bind directly to components of the COPII coat, primarily Sec24p,20,21,35 soluble cargo (and some transmembrane cargo) proteins interact with the COPII coat indirectly via an intermediary ER transmembrane adaptor, termed a cargo receptor, that in turn binds directly to the COPII coat.36-38 Most ER transmembrane cargo and cargo receptors bind to Sec24p, which contains at least 3 cargo binding sites, although Sar1p and SEC23 may also play a role in transmembrane cargo selection.20,22,23,39-41
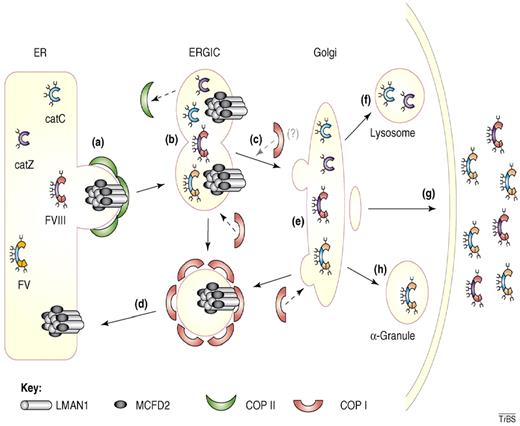
While multiple cargo receptors have been described in yeast,37,38,42,43 only 1 clear cargo receptor, the LMAN1-MCFD2 protein complex, has been defined in mammals.4,5,44 As discussed in further detail under “Pathogenesis of F5F8D,” the LMAN1-MCFD2 complex forms a cargo-specific receptor for coagulation factors V and VIII (Figure 2). The presence of orthologs for LMAN1 and MCFD2 in species that lack orthologs for FV and FVIII, such as Drosophila melanogaster and Caenorhabditis elegans, suggested that LMAN1-MCFD2 might also serve as a cargo receptor for additional proteins. Indeed, in vitro studies suggests that LMAN1 acts as a cargo receptor for 2 lysosomal proteins, cathepsin C (CatC) and cathepsin Z (CatZ), and for α1-antitrypsin.36,46,47
Cargo transport by the LMAN1-MCFD2 complex. (A) Factor V (FV), factor VIII (FVIII), cathepsin C (CatC), and cathepsin Z (CatZ) bind the LMAN1-MCFD2 complex and are packaged into COPII vesicles. (B) COPII vesicles bud from the ER membrane and fuse together forming the ER-Golgi intermediate compartment (ERGIC). (C) Anterograde transport of cargo protein to the Golgi apparatus occurs along microtubules. (D) The LMAN1-MCFD2 complex is recycled back to the ER through COPI vesicles. (E) Posttranslational modification of the cargo proteins occurs in the Golgi apparatus. (F) CatC and CatZ are transported to the lysosomes. (G) FV and FVIII are secreted outside of the cells. (H) In murine megakaryocytes, FV is transported and stored in α-granules. Reprinted from Baines and Zhang45 with permission.
Cargo transport by the LMAN1-MCFD2 complex. (A) Factor V (FV), factor VIII (FVIII), cathepsin C (CatC), and cathepsin Z (CatZ) bind the LMAN1-MCFD2 complex and are packaged into COPII vesicles. (B) COPII vesicles bud from the ER membrane and fuse together forming the ER-Golgi intermediate compartment (ERGIC). (C) Anterograde transport of cargo protein to the Golgi apparatus occurs along microtubules. (D) The LMAN1-MCFD2 complex is recycled back to the ER through COPI vesicles. (E) Posttranslational modification of the cargo proteins occurs in the Golgi apparatus. (F) CatC and CatZ are transported to the lysosomes. (G) FV and FVIII are secreted outside of the cells. (H) In murine megakaryocytes, FV is transported and stored in α-granules. Reprinted from Baines and Zhang45 with permission.
Combined FV and FVIII deficiency
F5F8D is an autosomal recessive disorder first described by Oeri et al in 1954.48 F5F8D is a rare disease, yet it is the most common form of familial multiple coagulation factor deficiency. To date, more than 80 families with this disorder have been reported, the majority from the Mediterranean region. F5F8D appears to be particularly prevalent in Jews of Sephardic and Middle Eastern origins49 and in non-Jewish Iranians,50 with a disease frequency of ∼ 1 in 100 000 persons. F5F8D is likely underdiagnosed as a result of the generally mild bleeding manifestations, and may also be misdiagnosed as either mild hemophilia A (FVIII deficiency) or parahemophilia (FV deficiency).
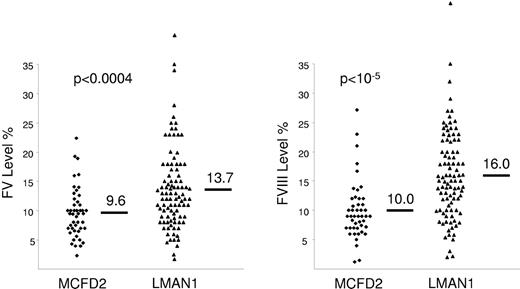
In F5F8D, FV and FVIII antigen and clotting activity levels are concordantly decreased, and result in prolonged prothrombin time and activated partial thromboplastin time. The factor levels are typically in the 5%-30% range,51 though levels as low as 1%-2% have been reported.50,52 The mean levels of FV and FVIII are slightly lower in patients with F5F8D due to MCFD2 mutations compared to patients with LMAN1 mutations.51 The difference, however, is not relevant for diagnostic purposes because of considerable overlap between both groups (Figure 3). In F5F8D, all other factor levels and routine blood tests are within the normal range. Based on these laboratory results, the differential diagnosis of F5F8D includes 2 other diseases, coinheritance of FV deficiency with either von Willebrand disease (VWD) or hemophilia A. F5F8D can be distinguished from coinheritance of FV deficiency and VWD by the results of VWD-specific tests. Coinheritance of FV deficiency and hemophilia A is extremely rare and may be evident from the complex inheritance pattern. In F5F8D, a positive family history may not be evident, but if present, should be consistent with an autosomal recessive inheritance.
Factors V and VIII levels in patients with F5F8D. Patients with F5F8D secondary to MCFD2 mutations have slightly lower FV and FVIII levels compared to patients with LMAN1 mutations, though there is significant overlap between both groups. Reprinted with permission from Zhang et al.51
Factors V and VIII levels in patients with F5F8D. Patients with F5F8D secondary to MCFD2 mutations have slightly lower FV and FVIII levels compared to patients with LMAN1 mutations, though there is significant overlap between both groups. Reprinted with permission from Zhang et al.51
Patients with F5F8D typically exhibit no or mild bleeding symptoms,49,50 most frequently including epistaxis, menorrhagia, and excess bleeding after tooth extraction, surgery, laceration, or delivery.49,50 Hemarthroses and bleeding after circumcision are less common, but their frequencies seem to vary in different populations.49,50 Gastrointestinal bleeding, hematuria, and CNS bleeding are uncommon. Severe and life-threatening bleeding is rare. Heterozygotes for F5F8D are asymptomatic and exhibit normal levels of FV and FVIII.
Therapy for patients with F5F8D should be individualized. Prophylactic therapy (except before procedures) is generally not recommended, and treatment should be reserved for patients who are bleeding.53,54 The treatment approach depends on the levels of FV and FVIII and the clinical presentation, and generally aims to increase both FV and FVIII levels.53
Pathogenesis of F5F8D
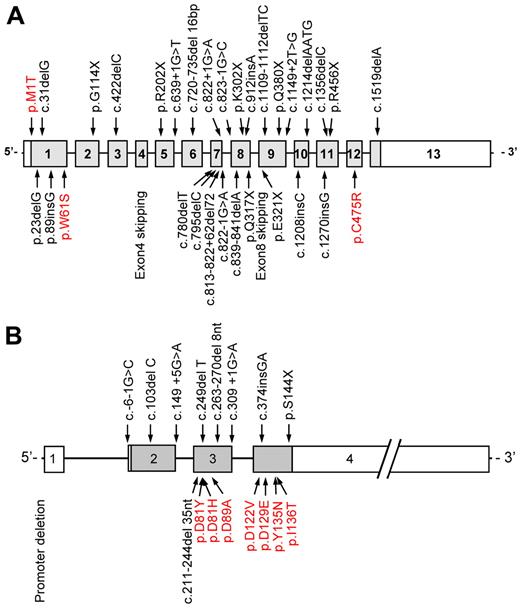
Decreased activated protein C (aPC) inhibitor activity in the plasma of patients with F5F8D was reported in 1980, suggesting that this disorder resulted from unopposed aPC activity on its substrates FV and FVIII. However, subsequent studies demonstrated normal levels of aPC inhibitor in these patients.55-58 The molecular pathogenesis of F5F8D then remained a mystery until advances in genetic methodology facilitated positional cloning, with genetic analysis of large pedigrees identifying 2 founder mutations in the LMAN1 gene. LMAN1 encodes a protein previously known as ERGIC-53 (ER-Golgi intermediate compartment 53-kDa protein).4 The LMAN1 or ERGIC-53 protein was known to mark the ERGIC, but its function was not known. LMAN1 gene mutations account for ∼ 70% of the cases of F5F8D. The MCFD2 gene was subsequently shown to account for all the remaining cases.5,44 To date, at least 33 different LMAN1 mutations (including 3 missense mutations) and 17 different MCFD2 mutations (including 7 missense mutations) have been reported59 (Figure 4).
Distribution of LMAN1 and MCFD2 mutations. At least (A) 33 different LMAN1 mutations and (B) 17 different MCFD2 mutations have been reported. Missense mutations are depicted in red. Reprinted from Zhang et al59 with permission.
Distribution of LMAN1 and MCFD2 mutations. At least (A) 33 different LMAN1 mutations and (B) 17 different MCFD2 mutations have been reported. Missense mutations are depicted in red. Reprinted from Zhang et al59 with permission.
LMAN1 is a 53-kDa protein consisting of an N-terminal luminal carbohydrate recognition domain (CRD) that binds mannose, a transmembrane domain, and C-terminal cytoplasmic ER exit and retrieval motifs.45,60-63 MCFD2 is a 16-kDa soluble protein that contains an N-terminal signal sequence and 2 calcium-binding EF-hand domains at the C terminus.64 LMAN1 and MCFD2 form a calcium-dependent complex in a 1:1 stoichiometry.65 The LMAN1-MCFD2 complex cycles back and forth from the ERGIC/Golgi to the ER.66 LMAN1 contains a Lys-Lys ER retrieval signal that is recognized by the COPI coat for retrograde transport.63 The C terminus of LMAN1 also contains a Phe-Phe exit motif that is recognized by the COPII coat for anterograde transport.62 Proper LMAN1 trafficking requires oligomerization of the protein.67 This is also important for MCFD2 binding.68 MCFD2 lacks a KDEL ER retrieval signal, and therefore relies on its interaction with LMAN1 for proper trafficking. In the absence of LMAN1, MCFD2 is not recycled back to the ER and is instead secreted from the cell.
Both LMAN1 and MCFD2 directly bind FV and FVIII.68,69 LMAN1 binds these 2 clotting factors through its CRD, while MCFD2 binds them through the EF-hand domains. LMAN1 and MCFD2 also interact with each other through CRD and EF-hand motifs70,71 ; however, the specific sites of interaction are distinct from the binding sites for FV and FVIII. LMAN1-deficient mice demonstrate decreased FV and FVIII levels (∼ 50% of normal), and slightly distended ER with accumulation of α1-antitrypsin in hepatocytes, though α1-antitrypsin plasma levels are not reduced.9
Congenital dyserythropoietic anemia type II
The congenital dyserythropoietic anemias (CDAs) form a group of rare hereditary disorders collectively characterized by ineffective erythropoiesis and specific morphologic abnormalities of the erythroblasts in the bone marrow (BM). Traditionally, CDAs have been classified into 3 major types (CDA I, II, and III).72 Recently, additional variants have been described.73
CDAII, also known as hereditary erythroblastic multinuclearity with a positive acidified-serum lysis test (HEMPAS), is the most common form of CDA. The prevalence of CDAII is not known, but more than 300 cases have been reported.74 CDAII may be diagnosed in children as well as in adults. In one report, age of diagnosis was as young as 1 month to as old as 78 years (median, 18.2 years).74 CDAII is an autosomal recessive disease clinically characterized by anemia, jaundice, and splenomegaly. The anemia is typically mild to moderate (median hemoglobin of 9.1-9.8 g/dL),74 and the majority of patients are not transfusion-dependent. Only ∼ 10% require regular red cell transfusions in infancy and childhood,74 and CDAII presenting as hydrops fetalis has been reported.75 The mean corpuscular volume is typically normal, though it may rarely be low or high. The white blood cell and platelet counts should be in the normal range. Typical peripheral blood smear findings include anisopoikilocytosis, occasional basophilic stippling, and rarely circulating bi- or multinucleated erythroblasts. The indirect bilirubin fraction is elevated in almost all cases of CDAII. Gallstones are common before the age of 40 years (∼ 55% of patients).74 Approximately 55% of patients have splenomegaly at diagnosis, with most of the remainder developing splenomegaly later in life.74 Unlike patients with CDAIII, almost all patients with CDAII (and CDAI) develop iron overload unrelated to blood transfusions. Left untreated, this secondary hemochromatosis results in organ dysfunction.
There is significant overlap between the clinical manifestations of CDAII and some other hematologic diseases. Therefore, a correct diagnosis of CDAII relies largely on the specific BM morphology and additional tests summarized in the following paragraphs.74
A typical CDAII BM examination reveals erythroid hyperplasia with an increased erythroid to myeloid ratio of 3-10, and the presence of 10%-45% (median 20%) bi- or multinucleated erythroblasts (Figure 5A).74 In addition, pseudo-Gaucher macrophages may be seen.74 Finding bi- or multinucleated erythroblasts is neither specific nor diagnostic for CDAII, and can also be seen in CDAI.77 The percentage of bi- or multinucleated erythroblasts is typically less in CDAI (3%-7%), with asymmetric nuclei in CDAI compared with symmetric nuclei in CDAII. Internuclear chromatin bridging in 1%-3% of erythroblasts is a distinguishing feature of CDAI. By electron microscopy, CDAI erythroblasts show a characteristic “Swiss cheese” chromatin appearance, which is absent in CDAII. CDAII erythroblasts have a distinctive finding of “double membranes” on electron microscopy (Figure 5B), resulting from residual ER,78 which is ordinarily eliminated during normal erythropoiesis. CDAIII is distinguished by the presence of “giant” multinucleated (up to 12 nuclei per cell) erythroblasts and absence of the unique electron microscopic findings of CDAI or CDAII erythroblasts.
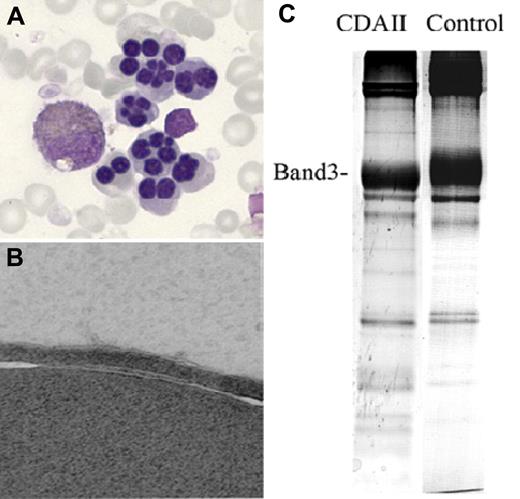
Abnormal findings in CDAII. (A) Bi- and multinucleated erythroblasts on BM light microscopy. (B) Erythroblast “double membranes” on electron microscopy. (C) Faster than normal shift of the RBC membrane band 3 protein on SDS-PAGE. Adapted from Denecke and Marquardt76 with permission.
Abnormal findings in CDAII. (A) Bi- and multinucleated erythroblasts on BM light microscopy. (B) Erythroblast “double membranes” on electron microscopy. (C) Faster than normal shift of the RBC membrane band 3 protein on SDS-PAGE. Adapted from Denecke and Marquardt76 with permission.
Additional tests may be useful in establishing a diagnosis of CDAII.74 Analysis of RBC membrane proteins by sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) typically reveals narrower band size and increased migration of the proteins band 3 and band 4.579-81 (Figure 5C), reflecting decreased protein glycosylation.
CDAII RBCs can also be identified by lysis in some acidified normal serum (Ham test). RBC lysis occurs because of the presence of an IgM Ab in normal sera that recognize an Ag present on the CDAII erythrocytes, but not on normal erythrocytes. A large number of different normal sera typically need to be tested to attain a reliable result, as only approximately one-third of random normal sera result in lysis of CDAII erythrocytes in an acidic milieu. This test is the basis for the initial description of this disease as HEMPAS.82
Treatment of patients with CDAII is largely supportive and aimed at preventing complications related to anemia and hemochromatosis. Patients with splenomegaly who are transfusion-dependent may sustain a significant increase in hemoglobin level postsplenectomy.74 Iron depletion is generally recommended before the ferritin level exceeds 1000 μg/L to prevent organ damage.74 The only curative option for CDAII patients is allogeneic BM transplantation.75,83,84
Pathogenesis of CDAII
The pathogenesis of CDAII has been a matter of controversy for decades. RBC membrane proteins, including band 3, band 4.5 and glycophorin A, were found to be consistently hypoglycosylated in CDAII.85 Conversely, lipids appeared to be overglycosylated.86 Some studies showed decreased activity of N-acetyl-glucosaminyltransferase II in CDAII,86-88 and one report showed reduced α-mannosidase II activity89 suggesting a defect in glycosylation. Based on these findings, a linkage study was performed using microsatellite markers flanking the genes N-acetyl-glucosaminyltransferease II, α-mannosidase II, and α-mannosidase IIx, though evidence for a specific locus at or near these genes was not identified.90
A zebrafish mutant was found that recapitulates some of the phenotypic features of CDAII including dyserythropoiesis and binucleated erythroblasts. The mutated gene was identified as scl4a1, which encodes the anion exchanger1 protein, also known as band 3.91 SCL4A1 was subsequently sequenced in 10 patients with mild CDAII, and no mutation was identified.91
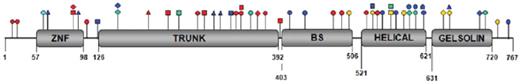
The CDAII locus was mapped by linkage analysis to a 5-cM interval on chromosome 20q11,76,92 and the responsible gene was recently identified as SEC23B11 . To date, at least 53 mutations have been described in 99 different patients, including missense, frameshift, nonsense, and splice site mutations.11,93,94 Patients have been identified as either homozygotes for the same mutation or compound heterozygotes for different mutations in SEC23B. The mutations in SEC23B are distributed throughout the gene and affect every domain of the protein (Figure 6), with no clear evidence for correlation of phenotype with specific genotype.94 No patient has yet been reported with 2 null SEC23B alleles, suggesting that complete SEC23B deficiency may be lethal. Patients with compound heterozygosity for a missense and a nonsense mutation may exhibit lower reticulocyte counts and higher ferritin levels than patients with 2 missense mutations, though there is considerable overlap between these 2 groups of patients.95 At present, it appears that most or all cases of CDAII result from biallelic SEC23B mutations.11,75,93-95 Although only a single heterozygous SEC23B mutation has been identified in some patients,93-95 it is likely that the second mutation is located outside of the sequenced region, such as in a distant regulatory sequence or a remote intronic sequence interfering with splicing.
Distribution of SEC23B mutations along the protein structure.SEC23B mutations affect every domain of the protein. The 4 different colors represent mutations reported in 4 different studies. ○ indicates missense mutation; □, nonsense mutation; ▵, splice site mutation; and ◊, frameshift mutation. Reprinted from Russo et al94 with permission.
Distribution of SEC23B mutations along the protein structure.SEC23B mutations affect every domain of the protein. The 4 different colors represent mutations reported in 4 different studies. ○ indicates missense mutation; □, nonsense mutation; ▵, splice site mutation; and ◊, frameshift mutation. Reprinted from Russo et al94 with permission.
How SEC23B deficiency results in the unique erythropoietic abnormality of CDAII remains a mystery. As noted previously, BM transplantation is curative for CDAII,75,83,84 suggesting that the erythroblast defect is intrinsic to the hematopoietic compartment. Multiple congenital dyserythropoietic disorders (reviewed in Iolascon et al96 ) share common features with CDAII. CDAI was recently shown to be due to mutations in the gene encoding CODANIN-1, a protein known to colocalize with SEC23B,97 suggesting a common pathophysiologic mechanism. However, a similar molecular link to other related dyserythropoietic disorders has not yet been identified.
Conclusion
Approximately 8000 proteins are transported from the ER to the Golgi apparatus via the COPII pathway, and the components of this system have been highly conserved throughout eukaryotic evolution. These observations might suggest that a defect in this transport system should result in a broad and multisystem phenotype. Contrary to this prediction, the reported mutations in multiple genes encoding the components of the early secretory pathway result in specific and limited phenotypes. Similarly, defects in several other basic biologic processes surprisingly result in limited phenotypes. For example, mutations in γ-glutamyl carboxylase, an enzyme that catalyzes posttranslational modification in multiple proteins, result in a phenotype explained largely or entirely by coagulation factor deficiency.59,98 Similarly, mutations in several genes encoding ribosomal proteins may result in Diamond Blackfan anemia, a disease characterized mainly by macrocytic anemia and low or absent erythroid precursors in an otherwise normocellular BM. These observations might suggest that the observed clinical phenotype for these disorders represents the “tip of the iceberg,” or the rate-limiting toxicity, with many other effects remaining subclinical.
Why mutations in SEC23B, a gene expressed in a wide variety of tissues,99,100 result in manifestations confined to the erythroid lineage remains unknown. A critical cargo required for erythroid differentiation and/or mitosis could depend specifically on Sec23B for its secretion. In zebrafish, scl4a1 gene mutation results in dyserythropoiesis and binucleated erythroblasts, suggesting that inefficient secretion of band 3, could be the basis of the disease.91 However, band 3 mutation in humans results in hereditary spherocytosis and similar disorders of RBC shape, not CDAII.101,102 Perhaps the SEC23A paralog overlaps in function with SEC23B and is widely expressed in other tissues, but not in sufficient quantities in erythroid precursors to compensate for the absence of SEC23B. Future identification of critical SEC23B-dependent cargos may explain the unique lysis of CDAII RBCs in only a subset of acidified normal sera, and the role, if any for hypoglycosylated membrane proteins in CDAII pathophysiology. SEC23B also interacts directly with SEC31, forming a link between the inner and outer layer of the COPII coat, and with Bet3 and p150Glued, components of the tethering complex TRAPPI and the dynactin complex, respectively,32,103 suggesting a role in vesicle tethering and transport along microtubules. How this relates to CDAII pathogenesis is unknown.
LMAN1-MCFD2 is the only well-established ER cargo receptor in mammals. While there is evidence for a few other soluble cargos trafficked from the ER by LMAN1 (α1-antitrypsin, CatC, and CatZ), the mechanism responsible for the transport of the majority of soluble secretory proteins from the ER to the Golgi remains unknown. Additional cargo receptors may be identified; alternatively, protein export from the ER could be—at least in part—unselective, consistent with the “bulk flow” hypothesis.104-106
Large and bulky cargos, such as extracellular matrix molecules and lipoprotein particles (100-500 nm in size), are considerably larger than COPII vesicles (60-80 nm).107 These macromolecules should not fit into standard COPII vesicles, and their mechanism of transport from the ER to the Golgi is puzzling. Intriguingly, secretion abnormalities for 2 macromolecules in this class, collagen and lipoproteins, are associated with genetic deficiency for specific COPII components (Sec24D in zebrafish and Sec23A and Sar1B in humans).
Finally, manipulating the COPII pathway may offer the potential for novel approaches to the treatment of CDA II and F5F8D, as well as several other disorders. The identification of the genetic basis for F5F8D also suggests LMANI and MCFD2 as potential novel therapeutic targets for anticoagulation. Complete deficiency of these proteins does not result in a general defect of COPII transport and produces only a mild bleeding phenotype, suggesting a wide margin of safety for this therapeutic approach.9
Acknowledgments
The authors thank Kärt Tomberg for assistance with Figure 1.
This work was supported by the National Institutes of Health (grant PO1 HL057346 and grant RO1 HL039693).
D.G. is a Howard Hughes Medical Institute investigator.
National Institutes of Health
Authorship
Contribution: R.K., M.P.V., and D.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Ginsburg, Life Sciences Institute, 210 Washtenaw Ave, Rm 5028, Ann Arbor, MI 48109-2216; e-mail: ginsburg@umich.edu.