Abstract
Acquired hemophilia A (AHA) is an autoimmune disease caused by an autoantibody to factor VIII. Patients are at risk of severe and fatal hemorrhage until the inhibitor is eradicated, and guidelines recommend immunosuppression as soon as the diagnosis has been made. The optimal immunosuppressive regimen is unclear; therefore, data from 331 patients entered into the prospective EACH2 registry were analyzed. Steroids combined with cyclophosphamide resulted in more stable complete remission (70%), defined as inhibitor undetectable, factor VIII more than 70 IU/dL and immunosuppression stopped, than steroids alone (48%) or rituximab-based regimens (59%). Propensity score-matched analysis controlling for age, sex, factor VIII level, inhibitor titer, and underlying etiology confirmed that stable remission was more likely with steroids and cyclophosphamide than steroids alone (odds ratio = 3.25; 95% CI, 1.51-6.96; P < .003). The median time to complete remission was approximately 5 weeks for steroids with or without cyclophosphamide; rituximab-based regimens required approximately twice as long. Immunoglobulin administration did not improve outcome. Second-line therapy was successful in approximately 60% of cases that failed first-line therapy. Outcome was not affected by the choice of first-line therapy. The likelihood of achieving stable remission was not affected by underlying etiology but was influenced by the presenting inhibitor titer and FVIII level.
Introduction
Acquired hemophilia A (AHA) is an autoimmune disease caused by an inhibitory autoantibody to factor VIII,1-4 which has an incidence of approximately 1.48/million/year.5 Well-recognized risk factors for AHA are malignancy, autoimmune diseases (systemic lupus erythematosus and rheumatoid arthritis), and pregnancy; however, approximately 50% of cases are idiopathic.2,5,6 The pattern of bleeding varies between superficial bruising that requires no hemostatic therapy in approximately one-third of patients to fatal bleeding, for example, intracranial, retroperitoneal, and gastrointestinal in 8% to 22%.5-7 Patients remain at risk of severe and fatal hemorrhage until the inhibitor has been eradicated, irrespective of the initial factor VIII level and inhibitor titer and even if they present with mild bleeding.5 For this reason, treatment guidelines recommend that patients are treated with immunosuppression as soon as the diagnosis has been made, with the aim of eradicating the inhibitor and normalizing the factor VIII level.8,9
First-line immunosuppression usually composes steroids alone or steroids plus cytotoxic agents (often cyclophosphamide),1,4 although there is increasing use of rituximab either alone or in combination with other agents.1,10-12 The outcome of immunosuppresive regimens depends on the efficacy of eradicating the inhibitor, the risk of relapse, and adverse events, including death. Information on adverse events is particularly important because the median age of patients with AHA is 77 years.13,14 There are major logistic challenges to undertaking randomized controlled trials in this disease area, and no adequately powered studies have been performed. The literature is composed of a single randomized study that showed no difference between treatment regimens but included an insufficient number of patients,15 case and single center reports (for reviews),1,4 and national surveys,4,5,16 but it remains unclear what the effect of standard immunosuppressive treatment is on inhibitor eradication and long-term survival. In the absence of randomized controlled trials, registry data from a large number of patients may provide useful information to guide clinical management.
Methods
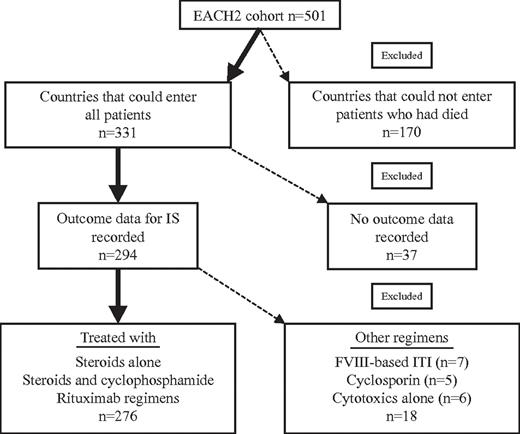
The European Acquired Hemophilia Registry (EACH2) collected data electronically on European patients with AHA between January 2003 and January 2009. A detailed description of the methodology and patient cohort has been previously published.14 The study was reviewed by ethics committees in each country, and informed consent was collected in accordance with the outcome of these reviews following the Declaration of Helsinki. In 2 countries, no informed consent was required; in 6 countries informed consent was required for patients who were alive but not those that had died; in 5 countries informed consent was required for all patients. In the latter 5 countries, patients who had died were, therefore, not recruited, and this is likely to have excluded a proportion of more severely affected cases. In this analysis, only persons from countries that could enter all patients have been included. In total, 501 patients were reported to the registry, of which 331 were from countries that could recruit all patients. In 37 patients, no outcome data were reported, leaving 294 patients in this report (Figure 1).
Disposition of patients from the EACH2 cohort included in this analysis of immunosuppression. The figure shows the EACH2 patient cohort and describes which patient groups were included in the analysis presented here.
Disposition of patients from the EACH2 cohort included in this analysis of immunosuppression. The figure shows the EACH2 patient cohort and describes which patient groups were included in the analysis presented here.
The outcome of first-line immunosuppressive therapy was analyzed in detail in 3 groups: steroids alone, steroids plus cyclophosphamide, and regimens based on rituximab (n = 276). These regimens could have been given orally or intravenously and at doses decided by the investigator. The primary outcome was induction of stable complete remission (CR). CR was defined as inhibitor undetectable and factor VIII more than 70 IU/dL, measured at the local laboratory, and immunosuppression stopped. Stable CR was defined as CR with no reported relapse during follow-up.
Statistical analysis
Descriptive results are expressed as median and interquartile ranges (IQRs) or frequency and percentage for continuous and categorical variables, respectively. Comparison between groups used the Mann-Whitney U test for continuous and the χ2 test for categorical variables.
To further investigate the effect of immunosuppression, a subset analysis was performed on those patients who received first-line treatment with oral prednisone alone compared with those who received oral prednisone and oral cyclophosphamide. To allow an unbiased comparison between the 2 groups, propensity score methodology17,18 was used. A logistic regression model was first assessed to predict the probability (propensity score) of receiving oral prednisone and oral cyclophosphamide or oral prednisone alone. This model included the following variables: factor VIII level and inhibitor titer at diagnosis, sex, age, weight, and etiology of AHA. Consequently, a 5 to 1 greedy 1:1 matching algorithm18 was used to determine matched pairs based on their propensity score. Adequacy of covariate balance in the matched sample was eventually assessed with McNemar or Wilcoxon signed rank test. The McNemar test was also used to assess differences between groups for the primary and secondary binary end points. Propensity score-matched models, adjusted for factor VIII at diagnosis, were further assessed. Binary end points were reported as odds ratio (OR) and 95% confidence intervals (CIs), whereas time-to-event end points were expressed as hazard ratios (HRs) and 95% CIs derived from a Cox proportional hazards regression model. Robust SEs were used both for logistic and Cox models to account for matched design. Time to event was plotted as Kaplan-Meier curves and compared with a log-rank test, for univariate analysis, and as Cox proportional hazards regression model estimated curves for PS-matched analyses.
To investigate whether the factor VIII level and inhibitor titer at diagnosis were useful for predicting outcome, survival c-index derived from a Cox model, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated.19,20 The survival c-index is the extension of the classic c-statistic for survival models. The c-statistic describes the proportion of pairs, comprising 1 subject with an event and 1 without, in which the model correctly predicts a higher probability of an event for the person who did experience it than for the person who did not. The IDI is a measure of the how much a parameter (eg, factor VIII level, inhibitor titer, or both) improves the overall predictive value of the model for inhibitor eradication compared with the baseline model and corresponds to the average over the range of all possible risk cut-offs of the improvement on sensitivity minus worsening on specificity. The NRI measures the improvement in prediction according to prespecified cut-offs of risk, namely, to assess the correct movement (upward for events and downward for nonevents) in predefined categories of the predicted values based on reclassification tables built separately for events and nonevents. The reference model was age, sex, and underlying etiology. This was compared with the reference model and presenting inhibitor titer (> or < 16 BU/mL), presenting factor VIII (as continuous variable), and both presenting inhibitor titer and factor VIII. The choice of including factor VIII as a continuous variable and inhibitor titer as categorical using its median value cut-off were derived from the best predictive model of each single predictor in a detailed analysis of all possible shaping of the variables themselves in the reference model, in which covariates were tested as continuous, and as median cut-off, tertiles, quartiles, and quintiles to account for a possible nonlinear relationship with the outcome at issue.
P values less than .05 were considered significant. All the analyses were performed using SAS Statistical Package release 9.1 (SAS Institute).
Results
A total of 501 patients were entered into the EACH2 registry; there were 331 from countries where all patients could be entered, and the outcomes of first-line immunosuppression were reported in 294 of these patients. Details of the immunosuppressive regimens used are shown in Table 1.
Steroids alone
There were 142 patients treated with steroids alone. Of these, 124 received oral prednisone at a median dose of 1.0 mg/kg (IQR, 0.92-1.07 mg/kg), 3 received dexamethasone, and 15 received other, unspecified steroids.
Steroids and a cyclophosphamide
There were 83 patients treated with a combination of steroids and cyclophosphamide. Of these, 81 received prednisone at a median dose of 1.03 mg/kg (IQR, 0.97-1.03 mg/kg), 1 dexamethasone, and in 1 case the steroid formulation was not reported. Cyclophosphamide was administered orally in 73 cases at a median does of 1.67 mg/kg (IQR, 1.3-2.1 mg/kg), intravenously in 9 at a median dose of 5.2 mg/kg (IQR, 2.9-10.6 mg/kg), and in 1 case the route was not reported.
Rituximab-based regimens
Rituximab was used in 51 patients in a wide variety of regimens. Rituximab alone was used in 12 patients and was combined with steroids, at a median dose of 1.0 mg/kg (IQR, 0.83-1.02 mg/kg), in a further 28 patients. Three patients received rituximab and a cytotoxic agent, and 8 had a combination of rituximab, steroids, and a cytotoxic agent.
Other regimens
Cyclosporine A and steroids were used in 5 patients, cytotoxic agents alone in 6, and regimens based on FVIII immune tolerance in 7. There were too few patients in these categories to be analyzed in detail, and these will not be reported further. Data on response to immunosuppression were not reported on 37 patients. Of the subgroup of 331 patients, 34 received intravenous immunoglobulin (IVIg) as part of their first-line immunosuppressive regimen, and outcome data were available on 33. The response to immunosuppression in a total of 276 patients is reported in detail in this analysis (Figure 1).
Patient characteristics dependent on first-line treatment
The median (IQR) for presenting age, factor VIII level, inhibitor titer and number with each etiology, and female/male ratio of all patients and the treatment subgroups are shown in Table 2. The presenting characteristics were similar, and there were no statistically significant differences between the groups.
Initial outcome of first-line treatment
Proportion achieving CR.
A higher proportion of patients treated with steroids and cyclophosphamide (66 of 83, 80%) achieved CR compared with those treated with steroids alone (83 of 142, 58%; Table 3). Of those treated with steroids alone, the median (IQR) time to a FVIII more than 70 IU/dL and undetectable inhibitor by Bethesda assay was 32 (15-51) and 34 (17-76) days, respectively (Table 3). Treatment with steroids and cyclophosphamide resulted in a FVIII more than 70 IU/dL and undetectable inhibitor by Bethesda assay at 40 days (18-81 days) and 32 days (12-77 days), respectively. CR (immunosuppression stopped) was reported after 108 days (55-208 days) in the steroid group and 74 days (52-151 days) in the steroid and cyclophosphamide group. Of the 51 patients treated with a rituximab-based regimen, 31 (61%) achieved a CR, the time to a factor VIII more than 70 IU/dL, and an undetectable inhibitor was 64 days (28-206 days) and 65 days (29-144 days), respectively, approximately twice as long as that observed for the other 2 groups. Because of variation in presenting characteristics and treatment regimens, it is not appropriate to perform statistical analyses to compare these groups directly.
Of the 33 patients treated with IVIg as part of their first-line immunosuppressive regimen for which outcome data were available, 15 of 33 (45%) achieved a stable remission and 18 of 33 (55%) did not. The treatment of this group was variable, but of the 15 treated with steroids alone and IVIg, only 2 (13%) achieved stable CR and 13 (87%) did not. Patients treated with other regimens, combinations of cytotoxic agents, rituximab and cyclosporine, 13 of 18 (72%) achieved a stable CR and 5 of 18 (28%) did not. These data cannot be further analyzed because of the low patient numbers.
Relapse and stable CR.
Relapse was reported in 15 (18%) patients who achieved a CR with steroids alone. This means that a stable CR after first-line treatment with steroids alone was reported in 68 of 142 (48%) patients. There were 8 (12%) relapses in the steroid and cyclophosphamide group, resulting in a stable CR in 58 of 83 (70%) patients. One patient relapsed after a rituximab-based regimen (3%), resulting in a stable CR in 30 of 51 (59%) patients (Table 3). The rituximab-based regimens, however, were very variable and cannot be interpreted as a single group; they are analyzed in more detail in “Rituximab-based first-line treatment.”
Time to relapse in the steroid-alone group was a median 134 days (IQR, 36-317 days) after the immunosuppression had been stopped. For the steroid and cyclophosphamide group, relapse was after 139 days (IQR, 14-135 days) and the single relapse after a rituximab based regimen occurred 44 days after CR. The median follow-up of the patients who achieved CR was 149 days (IQR, 30-603 days) after immunosuppression had been stopped.
Comparison of stable CR between treatment arms.
Initial descriptive analyses suggested that first-line treatment with steroid and cyclophosphamide resulted in a higher proportion of patients achieving a stable CR than treatment with steroids alone. There was, however, a wide variety of regimens, steroid formulations, routes of administration, and initial patient characteristics reported. These differences are likely to have influenced the observed outcomes and hence limit the value of a direct comparison between the 2 groups. Therefore, a more rigorous analysis was performed comparing patients treated with prednisone alone with those treated with prednisone and oral cyclophosphamide. The analysis was performed using patients matched by propensity score for age, sex, weight, presenting factor VIII level, and inhibitor titer and etiology. After optimal matching, there were 70 patients in each group, and the characteristics of the groups are shown in Table 4. The patients treated with rituximab-based regimens could not be compared by propensity score matching because there were insufficient patients and the variety of concomitant immunosuppressive agents used was too large.
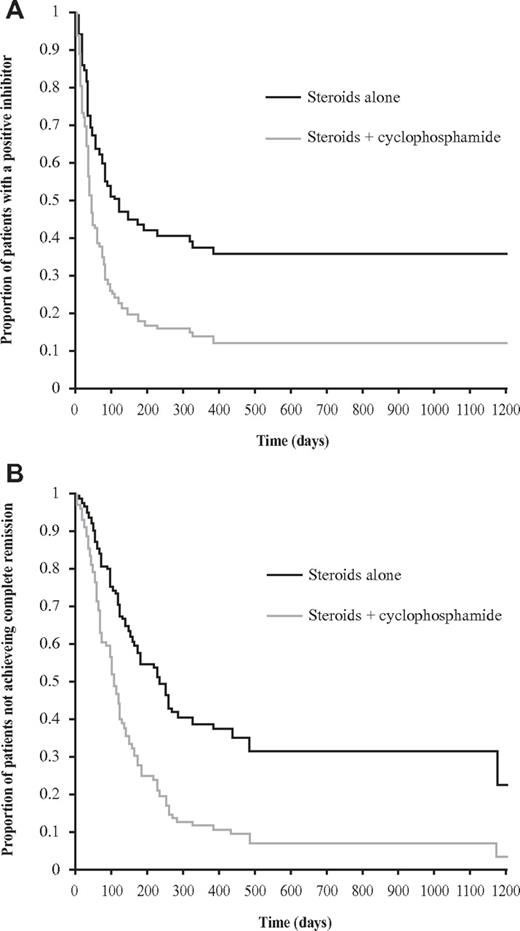
Propensity score-matched analysis showed that patients treated with prednisone and oral cyclophosphamide were more likely to achieve a stable CR than those treated with prednisone alone (OR = 3.25; 95% CI, 1.51-6.96, P < .003). Furthermore, in the matched groups, time to a negative inhibitor and normal factor VIII and time to CR (adjusted for presenting inhibitor titer) was shorter for the steroid and cyclophosphamide group compared with steroids alone (HR = 2.11; 95% CI, 1.38-3.21, P < .001 and HR = 2.36; 95% CI, 1.49-3.74, P < .001, respectively; Figure 2). These results were achieved despite the oral prednisone group being treated with a higher dose of steroids per body weight than the steroid and cyclophosphamide group, with a mean of 1.33 mg/kg (SD, 2.12 mg/kg) versus 1.18 mg/kg (SD, 0.38 mg/kg, P = .006).
Time to negative inhibitor of propensity score-matched groups and time to complete remission of propensity score-matched groups. Survival plots of propensity score-matched groups comparing steroids alone with steroids and oral cyclophosphamide (n = 70 in each group) showing: (A) time to negative inhibitor (HR = 2.11; 95% CI, 1.38-3.21; P < .001); and (B) time to complete remission (HR = 2.36; 95% CI, 1.49-3.74; P < .001).
Time to negative inhibitor of propensity score-matched groups and time to complete remission of propensity score-matched groups. Survival plots of propensity score-matched groups comparing steroids alone with steroids and oral cyclophosphamide (n = 70 in each group) showing: (A) time to negative inhibitor (HR = 2.11; 95% CI, 1.38-3.21; P < .001); and (B) time to complete remission (HR = 2.36; 95% CI, 1.49-3.74; P < .001).
Rituximab-based first-line treatment
Initial response to rituximab-based regimens.
There were 51 analyzable patients who received rituximab as part of their regimen; however, a wide variety of other immunosuppressants were used (Table 1), which makes it difficult to assess the effect of rituximab. Combining all of the rituximab-based regimens, 61% achieved remission, similar to steroids alone (58%) and less than the group treated with steroids and cyclophosphamide (80%). The 12 patients treated with rituximab alone had the lowest CR rate of any immunosuppressive regimen (42%). If patients who received rituximab and another agent were analyzed separately, then 67% achieved CR (Table 3).
Relapse was uncommon after a rituximab-based regimen (3%), and this meant that the proportion of patients achieving a stable CR was 59%, halfway between steroids alone (48%) and steroids plus cyclophosphamide (70%). The time to remission with rituximab was longer compared with other regimens, although immunosuppressive treatment was stopped sooner (Table 2).
Presenting characteristics and response to first-line immunosuppression.
To investigate the effect of baseline characteristics on the time between the initiation of first-line immunosuppression and inhibitor eradication and normal factor VIII (whichever occurred latest), a Cox regression model that included age, sex, and underlying etiology (idiopathic, autoimmune, malignancy, and pregnancy) was analyzed for the 240 patients for whom these data and outcomes had been recorded. The underlying etiology and sex were not significantly associated with time to inhibitor eradication and normal factor VIII, but age had a borderline significance: HR (95% CI) 1.02 (1.0-1.03, P < .03). Using Cox regression modeling, an inhibitor titer less than 16 BU/mL HR 1.57 (1.10-2.23, P < .02) and higher factor VIII level at presentation HR 1.06 (1.02-1.101, P < .001) were both associated with faster inhibitor eradication and normalization of factor VIII levels.
Compared with the baseline model (age, sex, and etiology), the addition of presenting inhibitor titer less than 16 BU/mL resulted in a survival c-index (95% CI) of 0.62 (0.58-0.67, P = .0006). The NRI for inhibitor titer less than 16 BU/mL was 15.75% (P = .14) and the IDI was 3.38% (P < .005). Factor VIII level at presentation resulted in a survival c-index of 0.61 (0.56-0.66, P < .02), NRI 16.48% (P = .08), and an IDI of 2.94% (P < .005). Combining both inhibitor titer less than 16 BU/mL and factor VIII level gave a survival c-index 0.64 (0.59-0.69, P < .001), NRI 16.48% (P = .06), and IDI 4.56% (P < .001) for the time to inhibitor eradication.
Adverse events associated with first-line immunosuppression.
Adverse events reported in association with each treatment arm are shown in Table 5. Among patients treated with steroids alone, 25% experienced at least 1 adverse event (most commonly infection, 16%), followed by diabetes and psychiatric disorders. A higher proportion of patients treated with steroids and cyclophosphamide experienced at least 1 adverse event (41%), compared with steroids alone, where more were reported to have experienced an infection (27%), and in 55% of these cases the infection was associated with neutropenia. The proportion of patients experiencing adverse events was similar comparing those that received oral cyclophosphamide (30 of 74, 41%) and intravenous cyclophosphamide (4 of 9, 44%). A similar proportion of patients treated with steroids alone and a combination of steroids and cyclophosphamide experienced diabetes and psychiatric disorders.
Of the patients treated with rituximab-based regimens, 37% experienced at least 1 adverse event. Diabetes was reported most commonly (22%), followed by neutropenia (18%) and infection (12%).
Subsequent therapies
Patients who did not achieve a stable CR with first-line immunosuppression were treated with a variety of second-line therapies, although data are unavailable for many patients. The second-line treatment received depended on first-line treatment and is shown in Table 6. In many cases, treatment involved the use of drugs to which the patients had not previously been exposed. Patients treated with first-line steroids were mainly treated with combination regimens, including cytotoxics or rituximab 50 of 63 (79%). Those treated first-line with steroids and cyclophosphamide were primarily treated with rituximab-based regimens: 13 of 20 (65%).
In patients for whom data are available and who had not achieved a CR after first-line therapy, after second-line treatment 40 of 69 (58%) were in stable CR. Although a higher proportion of patients treated for relapse achieved a second CR, 12 of 14 (86%), a second relapse resulted in a similar stable CR rate of 9 of 14 (64%).
Of the 113 analyzable patients who had not achieved a stable CR after first-line treatment, 94 (83%) had an undetectable inhibitor at final follow-up (or at the time of death), although continuing immunosuppression was required to suppress the inhibitor in 23 of these cases and the inhibitor was still detectable in 19 cases. At final follow-up, 63 of the 126 (50%) patients for whom data were available and who had not achieved a stable CR after first-line therapy were alive and inhibitor-free.
Outcome at final follow-up
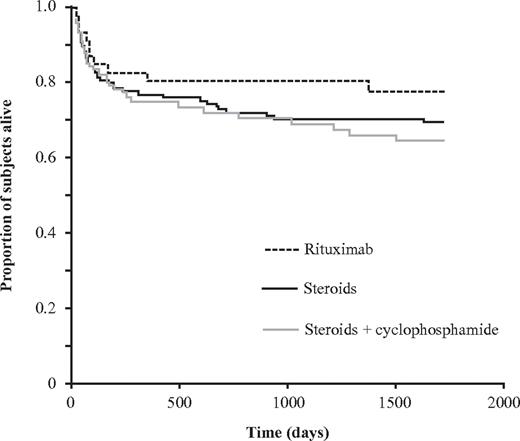
Of the whole group of 331 patients, survival data were available on 287 at final follow-up, which occurred after a median 262 days (IQR, 66-666 days), 198 (69%) were alive and 89 (31%) had died. Despite the lower proportion of patients achieving a stable CR with either steroids alone or a rituximab based-regimen, survival at final follow-up was similar for each group (Table 7; Figure 3). Of those treated with steroids alone for whom data were available, 36 of 128 (28%) patients were reported to have died. One patient died of bleeding, 5 were reported to have died of sepsis secondary to the immunosuppression, although none were neutropenic, and 6 died of an underlying malignancy. Of patients treated with steroids and cyclophosphamide, 22 of 66 (33%) for whom data were available had died. Of these, 4 were reported to have died of sepsis secondary to immunosuppression, and 1 of these patients was neutropenic. There were 4 patients reported to have died of a malignancy, and none died of bleeding. Of those treated with a rituximab-based regimen, 9 of 46 (20%) had died at final follow-up. Of these, 1 died of bleeding and 2 of the effects of immunosuppression; neither was neutropenic. Three patients died of an underlying malignancy. The other causes of death were predominantly of cardiac or respiratory disease or described simply as old age.
Proportion of patients surviving at final follow-up dependent on first-line immunosuppression. Kaplan-Meier plot showing the proportion of subjects (n = 287) who were alive at final follow-up dependent on first-line treatment after a median of 262 days (IQR, 66-666 days). There were no significant differences between the groups.
Proportion of patients surviving at final follow-up dependent on first-line immunosuppression. Kaplan-Meier plot showing the proportion of subjects (n = 287) who were alive at final follow-up dependent on first-line treatment after a median of 262 days (IQR, 66-666 days). There were no significant differences between the groups.
The proportion of patients who survived and were inhibitor-free at final follow-up was similar, although 15 patients required ongoing immunosuppression to sustain an undetectable inhibitor (Table 7). Patients who achieved a stable CR after first-line therapy were more likely to be alive at final follow-up: 103 of 137 (75%) compared with 67 of 102 (66%) who did not achieve a stable CR.
To compare the effect of first-line treatment on outcome at final follow-up, the propensity score-matched groups, adjusted for presenting inhibitor titer, were analyzed. This showed that the OR (95% CI) of being in CR at final follow-up comparing steroids and cyclophosphamide versus steroids alone was 3.16 (1.20-8.31, P < .02). In contrast, the OR of being alive was 0.91 (0.45-1.82), and being alive and in CR was 1.26 (0.64-2.48). Furthermore, time to death by Cox regression modeling was HR (95% CI) 0.93 (0.48-1.79).
Survival at final follow-up, however, was related to the presenting etiology. In pregnancy-related acquired hemophilia, the proportion of patients alive was 19 of 19 (100%), autoimmune 24 of 34 (71%), idiopathic 100 of 172 (58%), and malignancy 8 of 25 (32%).
Discussion
The results reported here compose the largest, most comprehensive, and most rigorously analyzed dataset investigating immunosuppression in acquired hemophilia A available to date. Previously, the total published literature has described the effect of immunosuppression with steroids and cytotoxics in 345 patients1 ; this study reports on 225 patients. A recent review on the role of rituximab described 65 patients,21 and this study reports on a further 51 patients. As such, the data described here contribute substantially to the published literature.
Although there are well-recognized shortcomings of registry data, the data derived are at least as good as the published literature, which consists of single-center cohorts, collections of experience from specialized centers, and national cohorts. The data in this registry are less likely to be affected by the bias of publishing good outcomes than other cohorts because centers entered consecutive patients prospectively. The centers were, however, predominantly specialist, and so referral bias is inevitable. Despite this, the very high number of patients reported strengthens the conclusions drawn. A number of steps were taken to minimize the potential biases associated with registry data. Only subjects from countries that could enter all patients were included, and specific treatment regimens were investigated only if sufficient patients had been entered. The variable length of follow-up is also an issue in an observational study, and some patients in the stable CR group may not have been followed for a long enough period to observe a relapse. Although prospective randomized studies are the best means with which to compare immunosuppressive regimens, the expense and difficulty of recruiting sufficient patients with acquired hemophilia A make this sort of study unlikely to be performed. The use of propensity score matching overcomes many of the shortcomings of registry data and is the most robust methodology available with which to investigate these questions if randomized studies are not possible or available.
Initial analysis suggested that steroids combined with cyclophosphamide resulted in more patients achieving a stable CR (70%) than either steroids alone (48%) or rituximab-based regimens (59%). The use of IVIg did not improve outcome, as has previously been shown.4,5,22 Because a variety of regimens were used, a more focused analysis was performed looking at prednisone alone compared with prednisone and oral cyclophosphamide. To control for possible factors that might affect outcome, the groups were propensity score-matched for age, weight, and presenting factor VIII level, inhibitor titer, and underlying etiology. This analysis confirmed that the combination of prednisone and cyclophosphamide was more likely to result in a stable CR and that this was achieved more rapidly. The results more than double the data available in the literature and agree with the findings of a literature review, which identified 20 studies and found that 89% of patients treated with cyclophosphamide achieved CR compared with 70% with steroids.4 A smaller study of consecutive patients presenting in the United Kingdom suggested that steroids alone and steroids and cytotoxics resulted in a similar proportion of patients achieving a CR of 76%.5 A more recent literature review reported a CR rate with steroids and cytotoxics of 78% and with steroids alone of 72%.1 This study, therefore, reports a lower rate of CR for steroids alone compared with the published literature but agrees with the CR rate previously reported for steroids and cytotoxics.
The best means with which to establish whether immunosuppressive regimens have different efficacy for inhibitor eradication is to perform a prospective randomized trial. To detect a decrease in stable CR from 70% to 50%, as observed in this registry between steroids and cyclophosphamide and steroids alone, it would be necessary to randomize 186 patients to have an 80% chance of detecting this difference with 90% probability. In the context of acquired hemophilia A, this would be a challenging undertaking, and in the absence of such trials, the registry data presented here, analyzed using propensity score, is the best available alternative.
Despite the discrepancy in the final CR rate between this and some previous studies, the median time to factor VIII normal and inhibitor undetectable observed in this study and the previously published literature was remarkably similar, at approximately 5 weeks for steroids alone and steroids plus cyclophosphamide.23 These studies, therefore, describe a reproducible time for patients to normalize their factor VIII level and no longer be at risk of abnormal bleeding. The data suggest that first-line regimens need to be observed for at least 5 weeks before a decision can be taken on whether any clinically useful effect has occurred.
The United Kingdom surveillance study had previously reported that a significant proportion of patients who achieved a first CR relapsed.5 For this reason, this study has reported stable remission after first-line immunosuppression as a more clinically relevant end point than CR. The data reported here confirm that relapse is relatively common after treatment with steroids, either alone or in combination with cyclophosphamide, but appears to be less common after a rituximab-based regimen. A similar proportion of patients were observed to relapse after steroids, with or without cyclophosphamide, in this study as was seen in the United Kingdom study (15% compared with 24%). Furthermore, in this study the median time to relapse is reported to be approximately 4 months, a very similar time to that reported in the United Kingdom study (median, 3 months).5 The reproducibility of these results strongly supports the assertion that relapse is an important feature of acquired hemophilia, and the findings highlight the importance of adequate follow-up, both in the clinical setting and when reporting the results of clinical studies. Very few studies in the literature have reported any relapses, and it is very likely, therefore, that relapses have occurred but not been observed and brings into question whether the reported results are a true reflection of the efficacy of the regimens described.
Rituximab is being used more commonly in patients with acquired hemophilia, and initial studies have suggested good results. A review of the literature reported on 65 patients treated with rituximab, and in many cases other agents, showed a CR rate of 90%.11 This response rate is substantially higher than the results reported here, and the bias toward reporting good outcomes is the most likely explanation, a possibility already highlighted by the authors of the review. In support of this assertion, of the 8 studies in the review reporting 100% CR, the number of patients included was between 2 and 4. The results reported here contrast with the published literature by showing that fewer patients achieved a stable CR than with steroids and cyclophosphamide or steroids alone. Indeed, the use of rituximab alone was the least likely regimen to achieve a stable CR. In contrast, a recent report of 12 patients treated with rituximab alone from a single center describes a 75% response rate.10 Although rituximab did not appear to show any advantage in achieving a first CR compared with other regimens, fewer relapses were observed; and so, in comparison, a relatively better stable CR rate was seen.
The rituximab-based regimens were also associated with a longer time to achieve a negative inhibitor and normal FVIII than other agents with a median time about twice as long. In the 12 patients treated with rituximab alone, the time to CR was 53 to 334 days. Boles et al report a median time of CR of 106 days (IQR, 25-185 days) in a further 12 patients treated with rituximab alone.10 It is possible that the slow response may in part be the result of the 3 to 4 weeks required to give the rituximab infusion regimen. However, given that 25% of untreated patients may have a spontaneous remission,7 it is possible that some of the late remissions seen with rituximab alone may also be spontaneous. This suggests that immunosuppression with rituximab alone may leave patients at risk of bleeding for longer than other regimens. The literature on rituximab is hard to interpret because of the wide variety of concomitant immunosuppressive agents used. The data presented here, however, do not support the use of rituximab alone or, if used as a first-line treatment, that it is better or safer than other less expensive immunosuppressive agents.
Salvage regimens were varied, but a significant proportion of patients were treated with rituximab as second-line therapy. The response to second-line treatment for relapse was more than 80%; although numbers were small, in the United Kingdom study the response rate was 63%.5 In this registry, second-line therapy after a failure of first-line treatment was successful in approximately 60% of cases. The effect of salvage regimens and side effects meant that, despite the different stable CR rates after first-line immunosuppression, survival at final follow-up was similar for each regimen, and this was confirmed in the propensity score match groups. Furthermore, the likelihood of being alive and inhibitor-free at final follow-up was similar between regimens, again confirmed after propensity score matching. This suggests that patients who do not respond to first-line therapy can be successfully rescued by subsequent treatment.
The data presented here confirm that immunosuppression in this patient group is associated with significant side effects, such as sepsis, neutropenia, diabetes, and psychiatric illness. The requirement to eradicate the inhibitor rapidly to reduce the risk of severe and fatal bleeding needs to be balanced with the risk of side effects in an elderly population, especially as fatal bleeding in the cohort, was uncommon at 3.2%.14 The development of less toxic immunosuppressive regimens would be an important step forward in AHA.
This study demonstrates that a presenting inhibitor titer less than 16 BU/mL and higher FVIII levels are associated with improved outcomes. This association was also observed in a prospective randomized study of 31 patients.15 This suggests that these markers describe an aspect of disease biology associated with response to immunosuppression. It is possible that adjusting treatment based on these parameters might be useful, but this will require further study; and at present, data are insufficient to adjust immunosuppression based on presenting factor VIII level and inhibitor titer.
In conclusion, these data show that patients with AHA are more likely to achieve a stable remission after first-line therapy if treated with a combination of steroids and cyclophosphamide than with other regimens. Despite this, outcome at final follow-up is not affected by the choice of first-line therapy. Stable remission was not affected by underlying etiology but was influenced by presenting inhibitor titer and FVIII level.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their collaborators in the participating centers for data acquisition and entry. Technical and registry database support was provided by Parexel International, Berlin, Germany. Editorial assistance was provided by Physicians World Europe, Mannheim, Germany, supported by Novo Nordisk Heath Care, Zurich, Switzerland.
The registry was supported by Novo Nordisk Region Europe A/S, Zurich, Switzerland (unrestricted educational grant).
Authorship
Contribution: P.C. and F.P. were primarily responsible for the statistical analyses; P.C. drafted the manuscript; and all authors participated in definition of data fields to be collected in the registry and the development of the electronic case report form, participated in the review, revision, and approval of the manuscript, and had access to all of the primary data.
Conflict-of-interest disclosure: Within the past 2 years, F.B. has received honoraria directly from Bayer, Baxter, Grifols, and Novo Nordisk. P.C. has served as a consultant for Novo Nordisk, Baxter Healthcare, CSL Behring, and Inspiration Pharmaceuticals and has received honoraria directly from Novo Nordisk, Baxter Healthcare, Bayer, CSL Behring, and Inspiration Pharmaceuticals. P.K. has served as a consultant, received research and travel funding, and has been a member of advisory committees for Novo Nordisk, Baxter, Archemix, and Ablynx. H.L. has served as consultant for Novo Nordisk and Baxter Healthcare and has received honoraria for lecturing from Novo-Nordisk. L.N. has received honoraria for lecturing and participation in advisory boards for Baxter, Novo Nordisk, and Pfizer. F.P. has served as a consultant for Novo Nordisk. P.M. has served as consultant and has been a member of advisory committees for Novo Nordisk. L.T. has served as a consultant for Ferring and Novo Nordisk and has received honoraria directly from them. A.H.-K. has served as a consultant for Novo Nordisk, Bayer, and Pfizer.
A complete list of the EACH2 registry contributors appears as a supplemental Appendix (available to the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Peter Collins, Arthur Bloom Haemophilia Centre, University Hospital of Wales, School of Medicine, Cardiff University, CF14 4XN Cardiff, United Kingdom; e-mail: peter.collins@wales.nhs.uk.