In this issue of Blood, Thon and colleagues provide “a novel live-cell imaging assay” to assess megakaryocyte differentiation and proplatelet formation, and demonstrate that trastuzumab-DM1 (T-DM1), a new cancer drug combination, affects both megakaryopoiesis and thrombopoiesis.1
Megakaryocyte formation (megakaryopoiesis) and subsequent platelet release (thrombopoiesis) are complex processes beginning with stem cells.2,3 Hematopoietic stemcells differentiate into immature megakaryocytes through lineage commitment stages, and they further differentiate into mature megakaryocytes with their highly unique features such as large size, polyploidism, and demarcation membrane system. Finally, mature megakaryocytes undergo terminal thrombopoiesis with proplatelet formation and platelet release.3
Understanding these processes have been complicated by asynchronous progression of megakaryopoiesis and thrombopoiesis over several days and by the admixture of noncommitted cells. When clinical thrombocytopenia is observed, the contribution made by altered megakaryopoiesis and thrombopoiesis may be hard to judge. Moreover, megakaryocytes and platelets have been differentiated from hematopoietic stem cells, fetal liver cells, embryonic stem cells, induced pluripotent stem cells, adipose tissues, and predipocytes.1,4-6 In part, these studies have been to elucidate the underlying mechanisms in megakaryopoiesis and thrombopoiesis, and have also been pursued to develop a donor-independent source for platelet transfusion.4 Because efficiency and the timing for differentiation of these various cell sources into megakaryocytes and then into platelets do approach 100%, these in vitro–generated megakaryocytes mature and release platelets asynchronously within a heterogeneous population of cells. Often, assessment of these in vitro–generated megakaryocytes have focused on morphology using Giemsa staining and electron microscopy, DNA polyploidy with nuclear staining, surface markers, and intracellular factor markers. These approaches have been useful, but are disadvantaged in that the studied cells are lost to subsequent sequential follow-up and in that it is difficult to do single-cell quantitative analysis.
The advantage of the new assay system by Thon and colleagues is that this system allows quantitative data analysis from individual cells under live culture conditions for extended periods of time (see figure). Its major disadvantage is that assay analysis is based on morphologic parameters. Combining this new assay with existing assays should allow more sophisticated linear analysis of the changes in progenitor cells as they commit to become megakaryocytes in a heterogeneous cellular background and/or cells at other points in commitment. Moreover, morphologic analysis at the level of sophistication provided by Thon and colleagues' system allows an analysis of the onset and degree of pro-platelet and formation over time. The stage of megakaryocyte maturation, point of platelet release, and degree of release in various systems can be both compared and optimized.
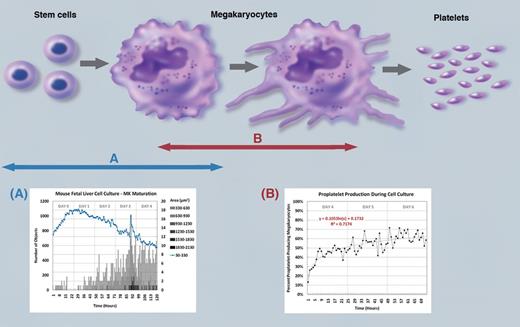
Process of platelet production from mature megakaryocytes through the differentiation of stem cells has complex and unique stages. The present study provides a novel assay system to quantitatively assess the levels of (A) megakaryocyte maturation from hematopoietic stem cells and (B) proplatelet formation from mature megakaryocytes under live culture conditions for extended periods of time. See Figure 2 in the article by Thon et al that begins on page 1975. Professional illustration by Marie Dauenheimer.
Process of platelet production from mature megakaryocytes through the differentiation of stem cells has complex and unique stages. The present study provides a novel assay system to quantitatively assess the levels of (A) megakaryocyte maturation from hematopoietic stem cells and (B) proplatelet formation from mature megakaryocytes under live culture conditions for extended periods of time. See Figure 2 in the article by Thon et al that begins on page 1975. Professional illustration by Marie Dauenheimer.
Drug-induced thrombocytopenia may be caused altering megakaryocyte development, platelet production, and/or clearance of circulating platelets. It is important to define the mechanism invoked by each drug. The authors demonstrate the use of their live-cell imaging assay system in a case of drug-induced thrombocytopenia caused by a human epidermal growth factor receptor 2–positive cancer drug T-DM1, a microtubule polymerization inhibitor.7 Microtubules have a critical role in proplatelet extensions. Thus, microtubule organization during proplatelet production was examined using the present assay system with a secondary fluorescent label for β-1-tubulin. These results showed an inhibitory effect of T-DM1, but not trastuzumab alone, on both megakaryocyte differentiation and proplatelet formation. The authors conclude from their analysis that DM1 inhibited proplatelet formation because of disrupting microtubule cytoskeleton.1
In summary, the present study provides a novel live cell imaging and analyses system that permits a time-profile quantitatively analysis of megakaryocyte maturation and proplatelet formation under live culture conditions for extended periods of time. This cell imaging is commercial available and the software to analyze the quantitative data are provided from National Institutes of Health. The authors described in detail how to integrate the software and the imaging system. Thus, the use of this assay system by Thon et al, should not only impact our understanding of megakaryopoiesis and thrombopoiesis, but the role of these processes and modification thereof in pathologic states.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■