Abstract
Over the past decade, extracellular nucleotides (such as ATP and UTP) have emerged as key immunomodulators. This family of molecules, already known for its key metabolic functions, has been the focus of intense investigation that has unambiguously shown its crucial role as mediators of cell-to-cell communication. More recently, in addition to its involvement in inflammation and immunity, purinergic signaling has also been shown to modulate BM-derived stem cells. Extracellular nucleotides promote proliferation, CXCL12-driven migration, and BM engraftment of hematopoietic progenitor and stem cells. In addition, purinergic signaling acts indirectly on hematopoietic progenitor and stem cells by regulating differentiation and release of proinflammatory cytokines in BM-derived human mesenchymal stromal cells, which are part of the hematopoietic stem cell (HSC) niche. HSC research has recently blended into the field of immunology, as new findings highlighted the role played by immunologic signals (such as IFN-α, IFN-γ, or TNF-α) in the regulation of the HSC compartment. In this review, we summarize recent reports unveiling a previously unsuspected ability of HSCs to integrate inflammatory signals released by immune and stromal cells, with particular emphasis on the dual role of extracellular nucleotides as mediators of both immunologic responses and BM stem cell functions.
Introduction
Living organisms are constantly exposed to foreign, and sometimes harmful, agents. To protect tissues from damage and preserve homeostasis, multicellular organisms have developed an array of defense responses of which inflammation is a major manifestation. As part of this defense mechanism, inflammation is instrumental for mounting an effective innate and adaptive immune response. Terminally differentiated cells (granulocytes, monocytes/macrophages, dendritic cells, B- and T-lymphocytes) have classically been considered the principal players in inflammation and immunity. Conversely, hematopoietic stem cells (HSCs), from which all immune and inflammatory cells derive, are usually thought not to be part of the immune system. Localized in the nurturing environment of the BM niche, HSCs were thought to reside within an “immunologic sanctuary,” protected from the insults affecting peripheral tissues. However, recent findings suggest that HSCs are not confined in a “splendid isolation”: BM-HSCs and circulating HSCs can sense the presence of danger or stress signals in the surrounding microenvironment and switch to an activated state or reach injured tissues in need of repair.1,2 Therefore, HSCs respond to early mediators of inflammation and distress that were so far thought to be active on immune cells only, such as TNF-α, IFNs, Toll-like receptor (TLR) ligands and, most interestingly, extracellular nucleotides (eNTPs).
From the evolutionary standpoint, nucleotides are among the most ancient biologic molecules; thus, it is not surprising that they have been used by living organisms for multiple purposes: storage and transmission of genetic information, energy metabolism, and extracellular communication.3 eNTPs compose both extracellular purines (ATP and its derivatives, ADP and adenosine) and extracellular pyrimidines (uridine-5′-phosphate [UTP] and [UDP]), which intervene in a variety of biologic processes by binding NTP-specific cell-membrane receptors, collectively named purinergic receptors. The presence of purinergic signaling in taxa as diverse as mammals, plants, yeasts and bacteria suggests that nucleotides are indeed an archaic, ubiquitous, communication system.4,5 However, their messenger role has been realized comparatively late, and only thanks to the pioneering studies of Geoffrey Burnstock in the central and peripheral nervous system.6 It is now well established that nucleotides mediate intercellular communication in virtually all tissues and typify one of the most important indicators of cell stress in the pericellular environment.7 In the hematopoietic and immune systems, eNTPs act as potent immunomodulators of neutrophil, monocyte, macrophage, dendritic cell, and T-lymphocyte responses.8 In addition, eNTPs drive blood cell proliferation and differentiation at different levels, including the compartment of hematopoietic progenitors and stem cells (HPSCs). Here we discuss how HSCs react to signals of cell injury and inflammation to mount an integrated response to pathogens and tissue damage. In our view, purinergic signaling activated by eNTPs emerges as a key element bridging inflammation to HSC activation.
How immunology blends into stem cell biology: the effect of immunomodulators on HSCs
Lying at the roots of the hematopoietic system, HSCs are a reservoir of rare, multipotent stem cells that provide a continuous supply of cells circulating in the peripheral blood (PB), such as erythrocytes, platelets, lymphoid cells, and myeloid cells. Under steady-state conditions, HSCs are highly quiescent cells that divide infrequently and are found mainly in the G0 phase of the cell cycle.9 Despite dormancy being the privileged status for HSCs, these cells retain a considerable resilience that enables them to adjust their cell-cycle dynamics and undergo proliferation when needed. Hemorrhagic stress is among the strongest triggers of HSC proliferation, aiming at replenishing the population of circulating erythrocytes lost through bleeding.10 Similarly, chemotherapic drugs (such as 5-fluorouracil or hydroxyurea), which kill proliferating hematopoietic progenitors, recruit HSCs out of quiescence to reconstitute the whole downstream subset of differentiated cells.11
Interestingly, increased HSC proliferation has also been observed in response to immunologic stress, such as infections. Under these conditions, HSCs skew their differentiation toward lymphoid and/or myeloid cells to maintain an efficient army of pathogen-fighting cells, until infectious agents are cleared. In analogy to stimulation of erythropoiesis by massive bleeding, pathogen-induced HSC proliferation has traditionally been interpreted as a physiologic reaction to infections, aimed at counterbalancing the loss of immune cells from PB. However, it is now clear that HSCs do not only react to the depletion of differentiated PB cells; rather, the HSC compartment reacts directly to danger signals and inflammatory cytokines released by pathogens, tissues, and resident immune cells. Several recent studies highlighted the role of IFNs as major regulators of HSC cell cycle.12-14 Both IFN-α and IFN-γ stimulate HSC proliferation in mice,14,15 and HSCs from IFN-γ-deficient mice are more quiescent and engraft better in transplantation assays than those from wild-type animals.12 Conversely, overactivation of IFN pathway impairs HSC functions and decreases their engraftment, suggesting a link between chronic infections and HSC exhaustion.16 The impact of infections on HSCs is also shown by the dual role of TNF-α on their proliferation and differentiation. Depending on the context, TNF-α plays inhibitory or stimulatory effects on HSCs.17,18 For instance, murine Tnfrsf1a−/− HSCs perform poorly in competitive repopulation assays because of self-renewal defects. On the other hand, excessive activation of TNF-α signaling is associated with impaired hematopoiesis,16 suggesting that any drastic imbalance in TNF-α signaling may be detrimental for HSC function.
HSCs sensing danger: the role of PRRs
Modulation of HSC functions by inflammatory cytokines is of great interest, but not novel and surprising by itself. More interesting and unexpected is the ability of HPSCs to respond directly to factors released by pathogens or by injured tissues, in other words to sense danger directly, unveiling a new skill for these cells and a potentially novel function in the activation of first-line immunity.1
Traditionally, immunology has focused on the self/non–self-discrimination. However, it is now gaining support the view that immune responses would be more efficient if discrimination was based not only on “foreignness” but also on “dangerousness,” as proposed by Matzinger's danger model.19 Multicellular organisms have evolved a wide array of extracellular receptors, named pattern recognition receptors (PRRs), specialized in recognizing pathogens or molecules released from injured cells (danger signals).20 PRRs bind viral or microbial pathogen-associated molecular patterns (PAMPs, such as lipopolysaccharide, peptidogycans, single-strand RNA, or unmethylated CpG motifs), and may also detect damage-associated molecular patterns. Damage-associated molecular patterns are endogenous molecules released by damaged or distressed tissues, may be of intracellular or extracellular origin, and include proteins (such as calgranulins) and nonprotein molecules (uric acid, reactive oxygen species, heparin sulfate, biglycans, tenascin C, and eNTPs).21 These molecules are collectively known as alarmins, a term recently coined to indicate endogenous molecules that alert immunity on tissue injury.22 Vertebrates possess a vast array of conserved PRRs (TLRs, RIG-I–like receptors, NOD-like receptors, AIM2-like receptors, and purinergic receptors), mostly expressed by monocytes, macrophages, neutrophils, and dendritic cells. Nonetheless, recent evidence indicates that also nonimmune cells, such as endothelial cells, and, more recently, HSCs express PRRs. These findings suggest that the network created by PAMPs, damage-associated molecular patterns, and PRRs transversally affects the whole hematopoietic system, from the compartment of immature HSCs up to the terminally differentiated immune cells.23,24 Through this signaling network, HSCs can directly sense a proinflammatory microenvironment even before inflammatory cytokines are released, driving a swift response to pathogens and accelerating the replenishment of circulating immune cells. As a direct consequence, repeated or chronic infections could negatively affect HSC long-term survival. As recently demonstrated in a model of chronic low-grade infection,25 the prolonged systemic exposure to TLR ligands is detrimental to long-term HSCs, thus highlighting a possible link between HSCs defects and different disease states, including infection, inflammation, autoimmune diseases, and aging.26-28
Purinergic danger signals and HSCs
Extracellular nucleotides as alarmins
Endogenous danger signals, collectively named alarmins, originate from cells or from the extracellular matrix. Those of cellular origin share a few fundamental features: (1) are rapidly released on necrotic cell death; (2) can be released either passively or through specialized efflux pathways; (3) recruit, by chemotaxis, target cells and activate innate and adaptive immune responses; (4) promote recovery from tissue injuries and restore homeostasis; and (5) their activity is counterbalanced by inhibitory pathways that avoid inflammatory loops.22 Purinergic signaling fulfils all these requirements, as nucleotides (1) are released into the extracellular environment on tissue injuries and cells death (passively or via specialized pathways)29 ; (2) stimulate immune cell chemotaxis and recruitment at inflammation sites by binding nucleotide-selective receptors, also known as purinergic receptors or P2 receptors (P2Rs)30-32 ; (3) activate innate and adaptive immune responses by stimulating antigen-presenting cells, neutrophils, monocytes- and macrophages8 ; (4) drive tissue recovery by stimulating cell proliferation33 ; and (5) promote resolution of the immune response by activating anti-inflammatory pathways dependent on ATP degradation products (eg, adenosine).34,35
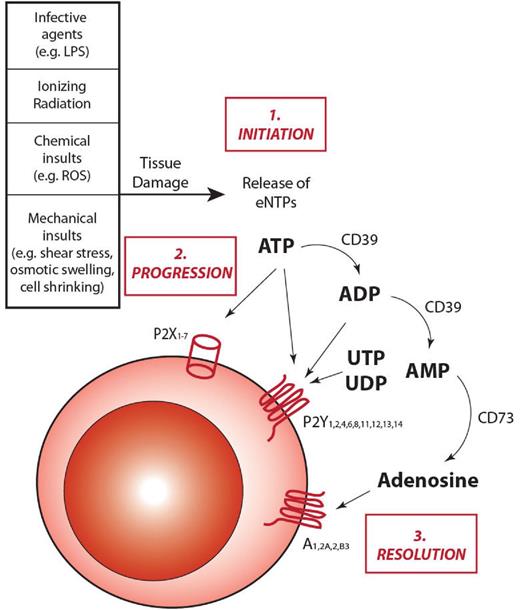
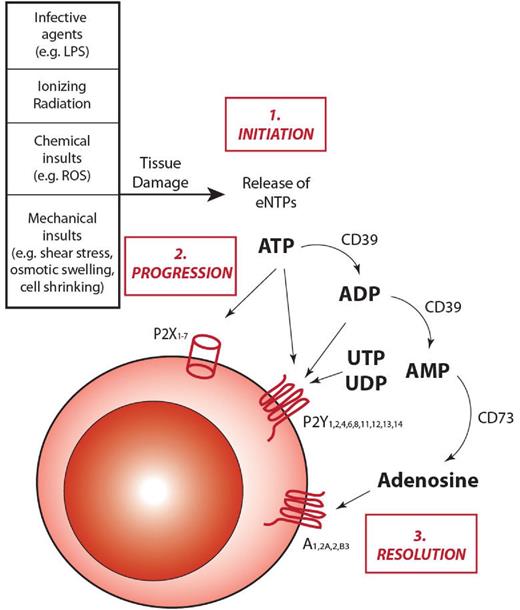
Similarly to other inflammatory cascades, purinergic signaling progresses through 3 phases: initiation, amplification, and resolution (Figure 1).
The role of extracellular ATP and purinergic signaling in inflammation. On insults inducing cell damage and stress, cells can release into the extracellular environment nucleotides, such as ATP. Once released, ATP can take part in all the different steps of inflammatory responses. 1. Initiation: after being secreted into the pericellular environment, ATP can act as a danger signal and alert the immune system, thus helping initiate the inflammatory response. 2. Progression: ATP can bind to P2 receptors exposed on the cell membrane of target cells and induce granulocyte and macrophage chemotactic attraction toward the inflammatory focus, as well as activation of antigen-presenting cells. These responses induce the activation of both the innate and the adaptive branches of the immune system, leading to the amplification of the inflammatory response. 3. Resolution: under physiologic conditions, inflammatory responses need to be restrained from self-amplifying without control. To protect tissues from extended damages, anti-inflammatory responses are activated. With specific regard to purinergic signaling, CD39 and CD73 endonucleosidases concur to down-regulate inflammation by hydrolyzing ATP in adenosine, with a double effect: prevent ATP from further activate P2R signaling and increase adenosine concentration, which activates anti-inflammatory responses by binding to P1 receptors.
The role of extracellular ATP and purinergic signaling in inflammation. On insults inducing cell damage and stress, cells can release into the extracellular environment nucleotides, such as ATP. Once released, ATP can take part in all the different steps of inflammatory responses. 1. Initiation: after being secreted into the pericellular environment, ATP can act as a danger signal and alert the immune system, thus helping initiate the inflammatory response. 2. Progression: ATP can bind to P2 receptors exposed on the cell membrane of target cells and induce granulocyte and macrophage chemotactic attraction toward the inflammatory focus, as well as activation of antigen-presenting cells. These responses induce the activation of both the innate and the adaptive branches of the immune system, leading to the amplification of the inflammatory response. 3. Resolution: under physiologic conditions, inflammatory responses need to be restrained from self-amplifying without control. To protect tissues from extended damages, anti-inflammatory responses are activated. With specific regard to purinergic signaling, CD39 and CD73 endonucleosidases concur to down-regulate inflammation by hydrolyzing ATP in adenosine, with a double effect: prevent ATP from further activate P2R signaling and increase adenosine concentration, which activates anti-inflammatory responses by binding to P1 receptors.
Initiation: release of eNTPs.
Several mechanisms responsible for nucleotide release into the extracellular fluids have been described so far. Extensive tissue injury, traumatic shock, or inflammation produces massive, nonspecific leakage of nucleotides because of cell lysis.8 However, nonlytic mechanisms are concurrently activated after stimulation with PAMPs. ATP, ADP, and other nucleotides are released via constitutive or stimulated exocytosis, as well as through poorly selective plasma membrane channels, such as connexins and pannexins.29 P2Rs themselves may participate in ATP release, as there is convincing evidence that the P2X7R subtype allows nucleotide efflux.36,37 It is now clear that virtually all cell types (hematopoietic cells included) constitutively release ATP to maintain a steady nucleotide concentration in the nanomolar range in the pericellular environment. However, different cell types release ATP in response to different stimuli (eg, erythrocytes in response to deformation, endothelial cells to shear stress, macrophage to endotoxin, osteoblasts, to mechanical loading). Thus, it is conceivable that eNTP release is a general signal of cellular stress, subsequently decoded by other cells to ignite inflammation.8,38
It is probable that a steady ATP level causes a tonic stimulation of P2Rs that is responsible for chronic, low-level, cellular activation. This is clearly shown by the in activity of ATP-hydrolyzing enzymes (eg, apyrase), which cause a decrease in cytoplasmic Ca2+ concentration or mitochondrial potential. Conversely, transient increases in eNTP concentration above steady-state levels are induced by several mechanical (shear stress, osmotic swelling, cell shrinking), chemical (reactive oxygen species, xenobiotics) or biologic agents (lipopolysaccharide)39,40 (Figure 1).
Although initial evidence dates back to several decades ago, it is only during the last 10 years that the role of ATP as extracellular messenger has been generally accepted despite some initial skepticism.41 One of the main arguments against the involvement of ATP in extracellular communication is the lack of an unequivocal in vivo demonstration that ATP is present in the extracellular space and that its levels are modulated during pathophysiologic responses. The very high cellular ATP content and its instability make ATP measurement very liable to sampling artifacts secondary to tissue manipulation. These experimental limitations have been finally overcome by developing ATP-sensing probes capable of detecting ATP in the pericellular space, where most of the biologic activity of eNTPs is localized.42,43 This innovative probe is a modified firefly luciferase engineered with a leader sequence and a glycosylphosphatidylinositol anchor, which enables routing and localization on the outer surface of the plasma membrane. Thanks to this bioluminescent ATP probe, pmeLUC (plasma membrane luciferase), we have been able to perform noninvasive sampling of eATP in vivo and to demonstrate conclusively that eATP is present at sites of inflammation and in tumor microenvironments.43 Similar detection assays for extracellular uridine nucleotides, based on the highly selective UDP-glucose pyrophosphorylase-coupled reaction, have been developed by other groups.44 This technical breakthrough paved the way for a systematic in vivo investigation of eATP involvement in inflammation and immunity and should also allow measurements in the BM. Average eATP concentration ranges under steady-state conditions in the 10−9-10−6M range. In proximity of the plasma membrane, it is expected to be higher, especially in protected pouches, which do not easily equilibrate with the pericellular space, but as of now it is very difficult to give accurate estimates. On cell stimulation, extracellular ATP levels increase to reach 10−4M40 (Figure 1). Although robust evidence shows that terminally differentiated erythroid, myeloid, and lymphoid cells release ATP, the precise pattern of eATP release in the BM microenvironment remains largely unknown: future studies measuring in situ eNTP concentrations in the BM are highly warranted.
Progression: activation of purinergic signaling.
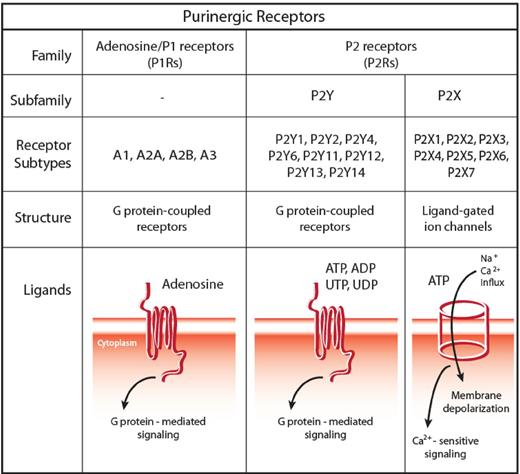
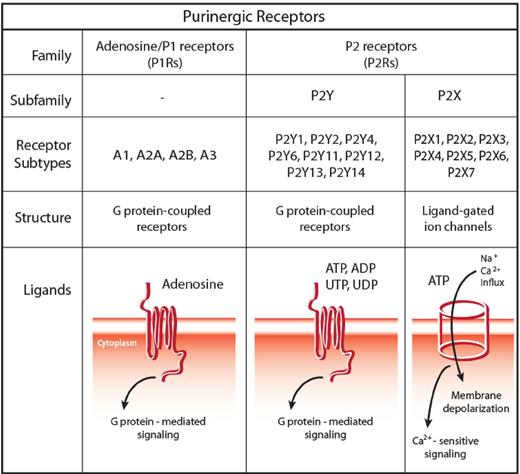
eNTPs bind to nucleotide-selective receptors, collectively named P2Rs. Based on pharmacologic profile and on molecular structure, 2 subfamilies have been identified, P2XRs and P2YRs.45 P2XRs (composing 7 subtypes, P2X1R through P2X7R) are trimeric ATP-gated plasma membrane channels.46 P2YRs are classic G protein-coupled receptors that, based on their ligand selectivity, are further subdivided in subtypes preferentially responding to ATP, ADP, UTP, UDP, or sugar-linked nucleotides, such as UDP-glucose and UDP-galactose30 (Figure 2).
The family of purinergic receptors: classification, structure, and signaling pathway. The family of purinergic receptors comprises 2 major groups of membrane receptors: adenosine-activated P1 receptors (P1Rs) and nucleotide-activated P2 receptors (P2Rs). Based on pharmacologic profile and molecular structure, 2 subfamilies have been identified within P2Rs, namely, P2XRs and P2YRs. P2XRs (composing 7 subtypes, P2X1R through P2X7R) are trimeric ATP-gated plasma membrane channels, whereas P2YRs are classic G protein-coupled receptors. Based on their ligand selectivity, P2YRs are further subdivided in subtypes preferentially responding to ATP (human and rodent P2Y1R, and human P2Y11R), to ADP (P2Y12R and P2Y13R), uridine-5′-phosphate (UTP) and UDP (human P2Y4R and P2Y6R), and ATP and UTP (human and rodent P2Y2R and rodent P2Y4R), whereas human P2Y14R binds sugar-linked nucleotides, such as UDP-glucose and UDP-galactose. Two different signaling cascades are associated with P2YRs: some subtypes mainly couple to Gq proteins (P2Y1R, P2Y2R, P2Y4R, P2Y6R, and P2Y11R), whereas others preferentially couple to Gi proteins (P2Y11R, P2Y12R, P2Y13R, and P2Y14R), with P2Y11R activating both molecular pathways.
The family of purinergic receptors: classification, structure, and signaling pathway. The family of purinergic receptors comprises 2 major groups of membrane receptors: adenosine-activated P1 receptors (P1Rs) and nucleotide-activated P2 receptors (P2Rs). Based on pharmacologic profile and molecular structure, 2 subfamilies have been identified within P2Rs, namely, P2XRs and P2YRs. P2XRs (composing 7 subtypes, P2X1R through P2X7R) are trimeric ATP-gated plasma membrane channels, whereas P2YRs are classic G protein-coupled receptors. Based on their ligand selectivity, P2YRs are further subdivided in subtypes preferentially responding to ATP (human and rodent P2Y1R, and human P2Y11R), to ADP (P2Y12R and P2Y13R), uridine-5′-phosphate (UTP) and UDP (human P2Y4R and P2Y6R), and ATP and UTP (human and rodent P2Y2R and rodent P2Y4R), whereas human P2Y14R binds sugar-linked nucleotides, such as UDP-glucose and UDP-galactose. Two different signaling cascades are associated with P2YRs: some subtypes mainly couple to Gq proteins (P2Y1R, P2Y2R, P2Y4R, P2Y6R, and P2Y11R), whereas others preferentially couple to Gi proteins (P2Y11R, P2Y12R, P2Y13R, and P2Y14R), with P2Y11R activating both molecular pathways.
Depending on tissue, engagement of P2XRs and P2YRs triggers a vast array of responses, ranging from cell proliferation or differentiation to necrotic cell death or apoptosis, from secretory exocytosis to chemotaxis, from reactive oxygen species generation to cytokine release. P2YRs respond to low nucleotide concentrations; thus, they are particularly suited to “sense” minute changes in nucleotide levels within the extracellular milieu and start chemotaxis. P2Y2R, P2Y6R, P2Y12R, and possibly P2Y13R appear to be the main subtypes involved in chemotaxis or inflammatory cell recruitment. Conversely, P2X7R seems to deliver a “stop signal” once cells have reached the core of inflammation.47 This is consistent with the different signals delivered by increasing ATP gradients: first, ATP activates high-affinity P2YRs and then the low-affinity P2X7R subtype, where ATP release and inflammation are higher. P2X7R plays an undisputed role in cytokine release. This receptor has emerged as a potent activator of the inflammasome and thus of IL-1β processing and release.48 Molecular details of this activity are however as yet obscure. The ability of eATP and P2X7R to promote inflammation has been established through several lines of in vivo evidence: of particular interest is their participation in GVHD. Like severe infections or traumas, GVHD leads to systemic inflammatory responses and causes tissue destruction and massive release of danger signals. In both humans and mice, ATP concentrations are increased during the development of GVHD, contributing to ignite a self-sustaining proinflammatory cascade involving IL-1α, IFN-γ production, donor T-cell expansion, and regulatory T-cell decrease.49
Resolution: down-regulation of purinergic signaling.
In the extracellular environment, nucleotides are also a substrate for nucleotide- and nucleoside-converting ectoenzymes, which rapidly hydrolyze ATP to ADP, adenosine-monophosphate (AMP), and eventually adenosine. The activity of ectoenzymes makes eNTPs very labile in the blood (and in general in the extracellular environment): consequently, eNTPs are mainly viewed as “short-range” mediators, although it is unlikely that they can act in an “hormone-like” fashion affecting responses of remote tissues and organs.
Enzymes known to hydrolyze nucleotides include the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family, the ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) family, the ecto-5′-nucleotidase, and alkaline phosphatases.40 NTPDase1/CD39 is one of the major nucleotide-hydrolyzing enzymes; and accordingly, CD39-null mice show clinical signs of exacerbated inflammatory responses, as seen in a model of skin inflammation secondary to chemical insults.50,51 The ecto-5′-nucleotidase CD73, which converts AMP into adenosine, is also critical in inflammation because of its anti-inflammatory/immunosuppressive activity. The anti-inflammatory role of CD73 is supported by in vivo experiments showing that CD73−/− mice develop enhanced inflammation and an accelerated graft-versus-host reaction.52 Anti-inflammatory effects of adenosine are mediated via a specific subfamily of purinergic receptors, named P1 receptors (ie, the A1, A2A, A2B, and A3 receptors.35 Interestingly, ATP is also a key modulator of immunosuppressive activity of T-regulatory lymphocytes. For a detailed description of ATP and adenosine function in immune cells, we refer readers to previously published reviews.53,54
Purinergic signaling in HPSCs
Purinergic signaling in hematopoiesis has been mostly investigated in terminally differentiated cells55 and found to participate in several cell functions, including platelet aggregation,56 chemotaxis,31,50 cell death, and proinflammatory activity,57 as summarized in Table 1. Historically, the investigation of purinergic signaling in hematopoietic cells has focused on ATP, UTP, and derivatives. On the contrary, little information is currently available on other purines or pyrimidines, reflecting the limited activity that nucleotides, such as CTP, GTP, ITP, and TTP, have generally displayed.30 Despite the large body of data on purinergic signaling in immune effector cells, investigation of eNTP-mediated responses on HPSCs started only a few years ago. Here, we summarize the effects of eNTPs on HPSC proliferation, differentiation, and migration.
Proliferation.
In 2004, we found that P2Rs are expressed on CD34+ hematopoietic progenitors and that their engagement produced fast changes in the intracellular calcium homeostasis. ATP and UTP enhanced the stimulatory activity of several cytokines on clonogenic CD34+ cells. Similar effects were also observed in lineage-negative CD34− progenitors, as well as in CD34+-derived long-term culture-initiating cells.58 Interestingly, stimulation with UTP increased the number of human BM-repopulating CD34+ cells after transplantation into immunodeficient mice.58 ATP-induced increase in the intracellular Ca2+ concentration also stimulated murine HSC proliferation and differentiation.59 More recently, eATP has been shown to reduce the number of HSCs as well as that of common myeloid progenitors and granulocyte myeloid progenitors, whereas mature BM myeloid cells were expanded. Interestingly, ATP-induced proliferation of HSCs resulted in a reduction in Notch expression, a marker of HSC quiescence.60
More recently, the link between ATP autocrine loops and HSC proliferation was investigated using the nucleotide-binding fluorescent probe quinacrine. ATP was found to actively accumulate within cytoplasmic vescicles in murine HPSCs.61 On stimulation of Ca2+-sensitive pathways, HPSCs can release these vesicles, igniting a positive autocrine loop that leads to P2Rs stimulation and further ATP release.61 Similarly to what previously observed,58 ATP positively affected cell-cycle dynamics in Lineage−c-Kit+Sca-1+ HPSCs in a cell-autonomous manner, with P2X1R and P2X4R subtypes most likely involved in the process.61 Finally, the role of endogenous ATP on HSCs becomes more prominent under inflammatory conditions. As assessed in 2 mouse models of T-cell-mediated chronic inflammation, ATP positively contributed to the expansion of both Lineage−c-Kit+Sca-1+ cells and phenotypically defined HSCs via the activation of P2XRs.61 However, inflammatory conditions have been shown to modify the phenotype of HSCs, potentially leading to imprecise conclusions. In the future, it will be important to address how purinergic signaling affects HSC ability to repopulate the BM in the context of inflammation.
The findings collected over the past decade suggest that purinergic signaling may promote the expansion of hematopoietic progenitors at the expense of more immature HSC subsets.60 Quiescence is fundamental for the long-term maintenance of hematopoiesis: a continuous stimulation of primitive HSCs with high concentrations of danger signals, such as eNTPs, may drive HSCs out of quiescence and lead to a premature exhaustion of hematopoiesis. In the future, knockout mice lacking P2 receptors or endonucleotidases are expected to provide new clues on the effects of eNTPs on HSC quiescence and long-term hematopoiesis. If confirmed, such a scenario may help understand the detrimental effect that chronic inflammation might have on long-term hematopoiesis (Figure 3).
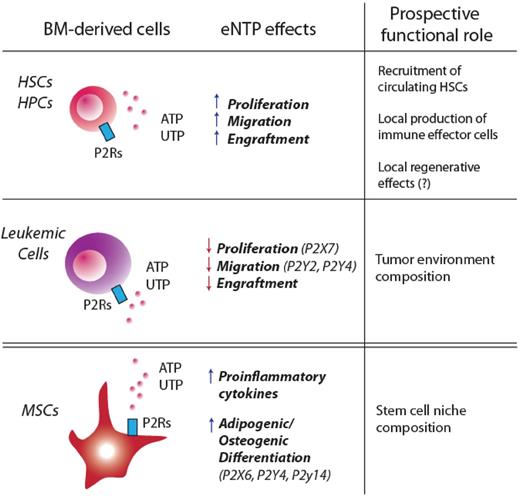
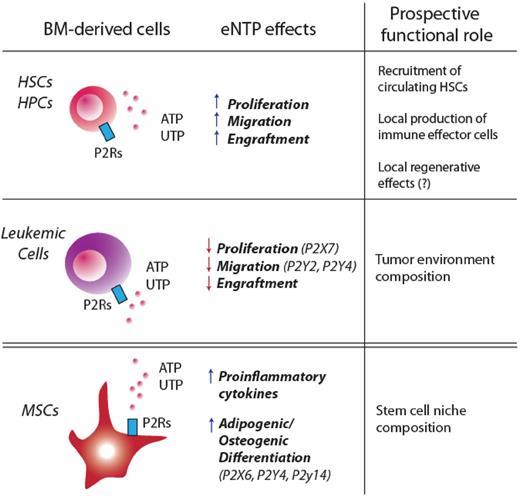
Purinergic signaling in BM-derived HPSCs, leukemic cells, and BM-derived MSCs. Recent findings suggest that purinergic signaling may affect HSCs at 2 different levels: by directly binding P2Rs on HPSCs or by targeting cells that are part of the HSC niche (such as MSCs). This figure summarizes how eNTPs directly modulate proliferation, migration, and engraftment in HPSCs and leukemic stem cells. BM-derived MSCs are also a target for eNTPs: activation of purinergic signaling in these cells has been shown to enhance their adipogenic/osteogenic differentiation, as well as the release of proinflammatory cytokines.
Purinergic signaling in BM-derived HPSCs, leukemic cells, and BM-derived MSCs. Recent findings suggest that purinergic signaling may affect HSCs at 2 different levels: by directly binding P2Rs on HPSCs or by targeting cells that are part of the HSC niche (such as MSCs). This figure summarizes how eNTPs directly modulate proliferation, migration, and engraftment in HPSCs and leukemic stem cells. BM-derived MSCs are also a target for eNTPs: activation of purinergic signaling in these cells has been shown to enhance their adipogenic/osteogenic differentiation, as well as the release of proinflammatory cytokines.
Differentiation.
Historically, investigations of purinergic signaling in hematopoiesis focused on lineage differentiation. Several reports contributed over the years to demonstrate the role of ATP in the differentiation of leukemia cell lines (HL-60 and NB4). Subsequently, new lines of evidence emerged supporting the widespread expression of P2Rs in hematopoietic cells. However, the pharmacologic profile of P2Rs sensibly differs among lineages and changes over time depending on the differentiation step. The differentiation of myeloid, erythroid, mekacaryocitic, and lymphoid progenitors has been shown to be influenced by eNTPs, although different receptors appear to be involved, depending on the specific lineage. For a detailed discussion on purinergic signaling in hematopoietic differentiation, we refer the reader to more comprehensive reviews on this topic.55,62
More recently, eNTPs have been found to have a tuning role on more immature myeloid progenitors. Barbosa et al showed that the in vivo administration of ATP depletes the number of granulocyte-macrophage progenitors in mice while increasing the fraction of mature (Gr1+, Mac1+) myeloid cells.60 Prospectively, this activity could be a direct response of the hematopoietic system to generic alarm signals, such as those released during infections. Besides accelerating the production of a larger army of immune cells from primitive progenitors, eNTPs can also provide a molecular bridge between cell damage and immune responses: ATP and UTP can act as “find-me” signals when released by apoptotic cells, helping recruit phagocytes and allowing clearance of dying cells from tissues.63
Migration.
Several reports suggested that molecules belonging to the circuit of innate immunity and regulating chemotaxis in immune cells may also play a role in enforcing HSC migration from the BM to peripheral tissues. Under physiologic conditions, HSCs are thought to primarily dwell in the BM, within the nurturing environment of the stem cell niche. The niche shields HSCs from external injury and helps maintain their survival, quiescence, and self-renewal. Nevertheless, HSCs have been found to circulate in the PB and other tissues as well. Although HSC peripheral traffic has been extensively characterized in clinical settings, their ability to egress the BM, circulate in peripheral tissues, and eventually home back to the BM under physiologic conditions is yet to be fully characterized. At any given time, between 100 and 400 HSCs are found circulating in the mouse PB64 : some of these reenter the BM, in a continuous exchange between the 2 compartments.65 It has been suggested that circulating HSCs are actually patrolling peripheral tissues, looking for potential injuries and infections.66 Lipopolysaccharide and other bacterial products have also been shown to easily reach the BM environment, where they stimulate TLR-expressing HSCs to produce more immune effectors. Nonetheless, migrating HSCs have an advantage over BM-resident HSCs: they may act as scouts in the peripheral tissues and sense the presence of pathogens directly at infection sites, possibly promoting a rapid and localized production of immune cells. For instance, complement cleavage-fragments released during immune responses have been shown to take part in HSC migration: C5 cleavage-fragments drive HSC mobilization, whereas C3 cleavage-products promote HSC retention in the BM. This finding has also been confirmed in animal models, with C5-deficient mice acting as poor mobilizers and C3-deficient mice mobilizing HSCs more efficiently.67 Such a scenario help envision HSC mobilization as an integral part of innate immunity.68
Recently, eNTPs have been shown to modulate HSC migration in the presence of CXC-chemokine 12 (CXCL12), considered the most important chemotactic factor for HSCs and responsible of stem cell retention in the BM niche. eUTP improves human HSC migration toward CXCL12 gradients and inhibits the down-regulation of membrane CXCR4 (CXCL12 receptor) in migrating CD34+ cells.69 Similarly, pretreatment with eUTP significantly increases BM homing of CD34+ HSCs when transplanted into immunodeficient mice.69 Of note, purinergic signaling can affect HPSCs also indirectly, by acting on the HSC niche. BM-derived mesenchymal stromal cells (BM-MSCs) represent a key component of the hematopoietic niche and secrete several HSC regulatory molecules, such as CXCL12 and stem cell factor. Recent findings showed that ATP treatment is associated with an increase in the production of proinflammatory cytokines in BM-MSCs (such as IL-2, IFN-γ, and IL-12p70), and the expression of purinergic receptors is modulated during adipogenic and osteogenic differentiation.70,71
Purinergic signaling in malignant hematopoiesis
It is becoming increasingly evident that tumor onset and progression depend not only on properties intrinsic to cancer cells. Both the biochemical composition of the tumor microenvironment and the cancer-host interaction appear to be important in disease evolution. eNTPs may now acquire a new biologic significance as part of the complex molecular miscellany composing the tumor environment.
Over the past decade, several in vitro studies described ATP's capability of suppressing cell growth in several cancer cell lines, including human HL-60 acute promyelocytic leukemia cells.72 In line with these findings, our group recently investigated the role of purinergic signaling in the tumor microenvironment associated with acute myeloid leukemia (AML). We found that AML cells express several functional P2XRs and P2YRs. Very interestingly, and conversely to HSCs from healthy donors,58 ATP inhibits cell proliferation and colony formation in AML cells, by restraining AML cells in G0, and reduces AML cell engraftment in immunodeficient mice.73
Among P2Rs, P2X7R appears to be a major mediator of ATP-dependent inhibition of cell growth in AML cells. As previously reported in chronic lymphocytic leukemia,74 also in AML P2X7 expression is higher, with highest levels in patients with a decreased remission rate. Remarkably, the highest P2X7R levels were detected in patients undergoing relapse, suggesting a role for this receptor in AML progression.75 P2X7R is also overexpressed in adult AML cells where its tonic stimulation promotes cell proliferation. Other P2XRs, such as P2X1R, P2X4R, and P2X5R are abnormally expressed in AML cells, suggesting a wide dysregulation and a possible pathogenic role of purinergic signaling in hematologic malignancies.76 On the contrary, ATP exerts an antiproliferative activity on leukemic blasts by down-modulating P2X7R expression.73 Based on this findings, eNTPs emerge as key components of the leukemic microenvironment where they appear to modulate proliferation and differentiation (Figure 3). In addition, eNTPs affect the motility of cells derived from AML patients.73 In migration assays, ATP inhibited the spontaneous migration of AML cells in vitro, whereas UTP also reduced CXCL12-driven migration. P2Y2R and P2Y4R receptors appeared to be the subtypes mainly involved in the process. Interestingly, AML BM homing and long-term engraftment are also decreased on exposure to eNTPs. Remarkably, ATP and UTP, as well as P2YR-agonists, significantly inhibited the long-term engraftment of highly purified CD38−CD34+ leukemic stem cells,73 suggesting that purinergic signaling can also affect rare leukemic stem cells, which usually escape most therapeutic approaches, making AML difficult to eradicate (Figure 3).
Translational implications and future perspectives
Translational studies on nucleotides are a rapidly expanding field that has prompted experimental therapeutic strategies against chronic inflammation and autoimmune diseases. In hematology, blocking purinergic signaling is now regarded as a promising pharmacologic approach for reducing the detrimental effects of GVHD without compromising graft-versus-leukemia responses.77 Purinergic signaling could be targeted at different levels: (1) by counteracting ATP release in response to chemical or infective insults; (2) by preventing the activation of P2Rs signaling cascades, such as the P2X7R-NALP3-inflammasome pathway; and (3) by enhancing ATP hydrolysis by ecto-enzymes (CD39 or CD73), promoting a quicker resolution of inflammation.
On the contrary, therapies enhancing purinergic signaling in the tumor microenvironment may prove useful in firing immunity against cancer cells.78 In addition, regulation of cell proliferation by nucleotides presents sensible differences between leukemic stem cells and HSCs: inhibition of AML proliferation and engraftment highlights novel routes of investigations for treating hematopoietic tumors while sparing healthy HSCs.
Deciphering what role purinergic signaling plays in vivo will also be critical for the mechanistic integration of eNTP effects on HSCs and the environment in which they reside. Because of the widespread expression of P2Rs, the biologic outcome of their stimulation will probably reflect the concerted activity on different cell subsets, including hematopoietic and nonhematopoietic cells.
New insights are also expected to arise from the role of purinergic signaling in bone physiology. Osteoblast cells play a pivotal role in the BM niche and osteoblast defects have previously been associated with impaired maintenance of HSCs. Interestingly, osteoblasts and osteoclasts are important sources of extracellular ATP, potentially affecting HPSC responses in the niche. In addition, several investigators reported bone deficiency in knockout mice lacking specific P2Rs. P2Y1R−/−, P2Y2R−/−, P2Y6R−/−, and P2Y13R−/− mice present bone abnormalities, and P2X7R−/− mice display a striking reduction in bone formation and remodeling,79 suggesting a role in balancing osteoclasts and osteoblast activity.80 Recently, studies in A2B knockout mice also highlighted a reduction in osteoblast differentiation, hinting at a role for adenosine in bone formation and resorption. Similar conclusions were drawn from the analysis of mice lacking CD73, suggesting that the disruption of adenosine signaling is highly detrimental to osteoblasts. Interestingly, CD73 blocking has been shown to significantly reduce tumor growth and metastasis.81 Of note, both BM-MSCs (from which osteoblasts are derived) and HPSCs (which give rise, among other lineages, to osteoclasts) express both CD39 and CD73,60 suggesting that new insights could arise from the pharmacologic targeting of endonucleosidases. If confirmed, purinergic signaling may emerge as a regulator of HSC-stromal cell interactions within the osteoblastic stem cell niche, and future studies may help elucidate the role of P2Rs in both steady-state and stressed hematopoiesis.
In conclusion, over the past 2 decades, a new picture of defense mechanisms in vertebrates has emerged. Not just specialized immune cells, but nonimmune cells as well may be involved, adding versatility and resilience to the whole system. Strikingly, some of these cells are primitive progenitors and stem cells, suggesting that immune effectors only represent the visible tip of a complex defense mechanism that deepens its roots into the stem cell compartment. In this view, the hematopoietic system no longer appears as a hierarchical model where the different compartments are hermetically organized. Instead, hematopoiesis is a dynamic system that reacts as a whole, where cells, either terminally differentiated or HSCs, speak a common language and react synergistically to protect the organism from danger and reestablish homeostasis (Figure 4). Several insults can subvert homeostatic conditions. Ionizing radiations, infections, chemical insults, and chemotherapeutic drugs all contribute to the release of nucleotides into the extracellular milieu. In the hematopoietic system, eNTPs can act at different levels: (1) by stimulating HPSC proliferation and differentiation, eNTPs and other alarmins contribute to immune cell production, invigorating defense mechanisms; and (2) circulating HSCs may sense danger signals coming from injured or inflamed tissues and migrate where immune effectors are needed the most, providing an in loco supply of granulocytes or antigen-presenting cells. Future insights may arise from other cell types participating to the healing of injured areas by responding to purinergic signaling, such as endothelial progenitor cells: in these cells, ATP has been shown to negatively affect proliferation and inhibit TLR4 expression, decreasing the release of proinflammatory cytokines.82
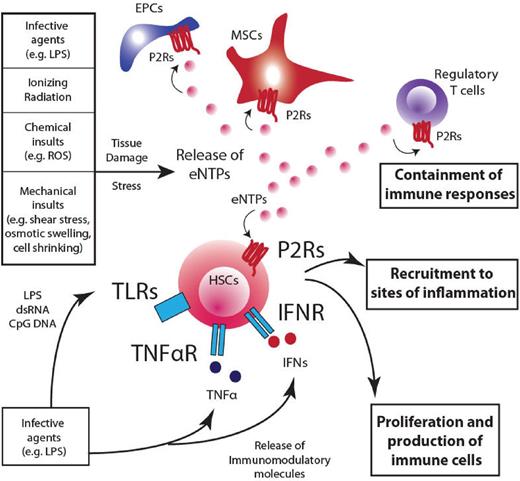
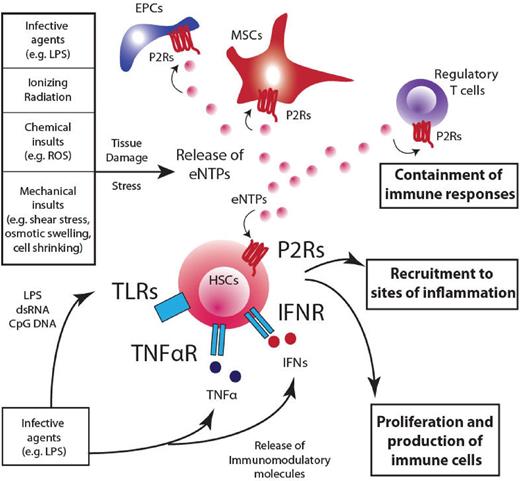
Extracellular nucleotides: pleiotropic players in the maintenance of homeostasis. A prospective overview of the role played by eNTPs in modulating responses to danger and how purinergic pathway integrates with immunomodulatory responses. Several insults can contribute to subvert the homeostatic condition in the hematopoietic system. Infective agents, ionizing radiations, chemical insults, and mechanical stress contribute to the release of nucleotides into the extracellular environment and the engagement of P2Rs on target cells (HSCs, regulatory T cells, MSCs, and endothelial progenitor cells), where eNTPs can cooperate in containing damage at different levels: (1) stimulate HSC proliferation and the subsequent production of immune effectors that will invigorate defense mechanisms; (2) attract HSCs, in synergy with other chemokines (eg, CXCL12), toward sites of inflammation and infection to activate tissue repair; and (3) contain detrimental effects of prolonged inflammation and immune responses acting on mature immune cells (eg, regulatory T cells). The net result of such a widespread effect on the hematopoietic system is aimed at damage containment and return to homeostatic conditions. EPC indicates endothelial progenitor cells.
Extracellular nucleotides: pleiotropic players in the maintenance of homeostasis. A prospective overview of the role played by eNTPs in modulating responses to danger and how purinergic pathway integrates with immunomodulatory responses. Several insults can contribute to subvert the homeostatic condition in the hematopoietic system. Infective agents, ionizing radiations, chemical insults, and mechanical stress contribute to the release of nucleotides into the extracellular environment and the engagement of P2Rs on target cells (HSCs, regulatory T cells, MSCs, and endothelial progenitor cells), where eNTPs can cooperate in containing damage at different levels: (1) stimulate HSC proliferation and the subsequent production of immune effectors that will invigorate defense mechanisms; (2) attract HSCs, in synergy with other chemokines (eg, CXCL12), toward sites of inflammation and infection to activate tissue repair; and (3) contain detrimental effects of prolonged inflammation and immune responses acting on mature immune cells (eg, regulatory T cells). The net result of such a widespread effect on the hematopoietic system is aimed at damage containment and return to homeostatic conditions. EPC indicates endothelial progenitor cells.
Acknowledgments
The authors thank Margaret A. Goodell for her valuable comments and suggestions.
This research was supported by the Italian Ministry for University Education and Research (PRIN 2008), Cassa di Risparmio di Bologna (project on Leukemic Stem Cells), the Italian Association for Cancer Research (IG 5354), Telethon of Italy (GGP11014), the Regione Emilia Romagna (Research Programs “Innovative approaches to the diagnosis of inflammatory diseases” and “Moniter”), and the University of Ferrara (institutional funds). L.R. was also supported by the Italian Leukemia and Lymphoma Association, section of Bologna (BolognaAIL).
The authors apologize to those authors whose work could not be referenced or discussed because of space limitations.
Authorship
Contribution: L.R., V.S., D.F., F.D.V., and R.M.L. designed and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lara Rossi, Department of Hematology and Oncological Sciences, L. and A. Seràgnoli, Institute of Hematology, University of Bologna, Orsola-Malpighi Hospital, via Massarenti, 9, I-40138 Bologna, Italy; e-mail: lara.rossi6@unibo.it