Abstract
This prospective study compared diagnostic and prognostic value of conventional cytologic (CC) examination and flow cytometry (FCM) of baseline samples of cerebrospinal fluid (CSF) in 174 patients with newly diagnosed aggressive non-Hodgkin lymphoma (NHL). FCM detected a neoplastic population in the CSF of 18 of 174 patients (10%), CC only in 7 (4%; P < .001); 11 patients (14%) were discordant (FCM+/CC−). At a median follow-up of 46 months, there were 64 systemic progressions and 10 CNS relapses, including 2 patients with both systemic and CNS relapses. Two-year progression-free and overall survival were significantly higher in patients with FCM− CSF (62% and 72%) compared with those FCM+ CSF (39% and 50%, respectively), with a 2-year CNS relapse cumulative incidence of 3% (95% confidence interval [CI], 0-7) versus 17% (95% CI, 0-34; P = .004), respectively. The risk of CNS progression was significantly higher in FMC+/CC− versus FCM−/CC− patients (hazard ratio = 8.16, 95% CI, 1.45-46). In conclusion, FCM positivity in the CSF of patients with high-risk NHL is associated with a significantly higher CNS relapse risk and poorer outcome. The combination of IV drugs with a higher CNS bioavailability and intrathecal chemotherapy is advisable to prevent CNS relapses in FCM+ patients.
Introduction
CNS dissemination in patients with aggressive non-Hodgkin lymphoma (NHL) is a relatively rare but often fatal complication that occurs in the different subtypes of NHL with a frequency of 5% (ie, diffuse large B-cell lymphoma [DLBCL]) to 30%1 (ie, Burkitt lymphoma [BL] and B-cell lymphoblastic lymphoma [B-LL]). Many CNS events occur early after diagnosis (4.7-9 months), during therapy, or shortly after completion of treatment, suggesting that initial CNS involvement could have been undetected in most cases. Prophylactic treatment likely reduces the incidence of CNS relapse, but may increase the toxicity of systemic chemotherapy; furthermore, the incidence of CNS dissemination is not high enough to suggest the use of prophylaxis treatment in all patients affected by aggressive NHL. Therefore, CNS prophylaxis is usually incorporated into protocols for the treatment of B-LL and BL, but it is not systematically warranted in patients with DLBCL. Therefore, the identification of patient subgroups for which CNS prophylaxis may be useful is important, especially for DLBCL patients who have no well-defined risk factors for CNS relapse. Risk models for DLBCL have been developed, but are mainly derived from analyses of retrospective studies.2–5 An increased risk of CNS dissemination is associated with involvement of the BM and certain extranodal sites such as testis, paranasal sinuses, orbits, and paravertebral masses. Moreover, patients with a high to intermediate or high risk according to the International Prognostic Index (IPI), particularly those with high serum levels of lactate dehydrogenase (LDH) and involvement of more than 1 extranodal organ, are much more prone to develop CNS involvement than others and should receive CNS prophylaxis.6 Nevertheless, CNS risk predictors and related prognostic scores have been identified in retrospective and heterogeneous series, including different lymphoma categories; however, resulting scores show a low sensitivity in predicting CNS involvement.
The diagnosis of CNS dissemination is frequently suspected by the presence of related signs or symptoms and confirmed by examination of cerebrospinal fluid (CSF) and neuroimaging techniques. The diagnostic standard conventional cytology (CC) examination of CSF is considered as having low sensitivity and low specificity, with reported false-negative rates of 20%-60%.7 This has been ascribed to the paucity of neoplastic cells in the CSF of patients with minimal disease and to the presence of confounding reactive lymphocytes. Therefore, patients with low tumor burden in the CSF, who are more likely to benefit from CNS treatment, are actually more commonly exposed to false-negative interpretations. Recent studies have demonstrated that flow cytometry (FCM) assessment of CSF could increase the proportion of positive cases detected with CNS dissemination compared with CC.8–14 However, it is still unknown whether detecting occult leptomeningeal involvement and consequently changing treatment may improve outcome in these patients.
In the present study, we compared prospectively FCM analysis versus CC examination of baseline samples of CSF to detect occult leptomeningeal disease in patients with aggressive B-cell NHL at high risk of CNS dissemination. We also evaluated the prognostic value, CNS recurrence rate, and therapeutic implications of detecting leptomeningeal involvement using both methods in these patients.
Methods
Study design
This is a multicenter, prospective, noncomparative trial on patients with newly diagnosed aggressive NHL at high risk for CNS dissemination conducted by the Fondazione Italiana Linfomi (FIL). The primary end point of this study was to compare the probabilities of detecting occult leptomeningeal involvement of FCM analysis on CSF samples versus standard CC examination. The secondary end point of the trial was to establish the value of FCM analysis in predicting CNS dissemination, progression-free survival (PFS), and overall survival (OS) in these high-risk patients.
The study was performed in accordance with the Declaration of Helsinki and was approved by the institutional review boards of the participating centers. All patients gave written informed consent. The trial was registered at www.clinicaltrials.gov as NCT00949741.
Patients
Between August 2004 and January 2010, 174 patients with newly diagnosed aggressive B-cell NHL at high risk of occult leptomeningeal dissemination were enrolled in 12 Italian centers. The inclusion criteria were as follows: (1) DLBCL with IPI scores of 2-3 and elevated LDH with involvement of at least 2 extranodal sites6 ; (2) DLBCL with involvement of BM, testis, paranasal sinuses, and/or orbits or with paravertebral masses with every IPI and LDH serum level6 ; (3) lymphoma categories with increased risk of CNS dissemination, such as BL, blastoid variant of mantle cell lymphoma (B-MCL), and B-LL; and (4) every aggressive lymphoma in HIV+ patients. Patients with clinical signs of neurologic disease were excluded from the study. The baseline assessment included physical examination, chest and abdomen computed tomography (CT) scans, BM biopsy, and a full laboratory workup. The CSF was obtained by lumbar puncture and was evaluated using standard methods, including glucose, total protein, evaluation for infectious organisms as appropriate, standard CC examination, and FCM.
Registered patients were managed with conventional chemoimmunotherapy according to lymphoma histotype: R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone/prednisolone)–like16 or R-MACOPB (rituximab, methotrexate, leucovorin, doxorubicin, cyclophosphamide, vincristine, and prednisone/bleomycin)17 for patients with DLBCL or B-MCL, and treatment protocols included high-dose CNS-penetrating systemic drugs such as high-dose methotrexate and high-dose cytarabine in R-HDS (rituximab-supplemented high-dose sequential chemotherapy)18 and R-CODOX-IVAC (rituximab-supplemented cyclophosphamide, vincristine, and doxorubicin plus ifosfamide, etoposide, and cytarabine)19 for BL, B-LL, and HIV+ lymphomas. Intrathecal (IT) prophylaxis chemotherapy was given as follow: patients with DLBCL and B-MCL received 12 mg of methotrexate with or without cytarabine weekly for a total of 4 doses and patients with BL or B-LL received IT liposomal cytarabine 50 mg every 2 weeks for a total of 4 doses. Patients with positive CC analysis of CSF were given liposomal cytarabine 50 mg every 2 weeks for a total of 5 induction doses plus 4 doses given monthly as maintenance. The IT drug delivery schedule was chosen irrespective of FCM results of CSF.
Cytologic examination
All samples were processed within 3 hours after collection. Cytospins were prepared by centrifuging 200 μL of fresh CSF at 14g for 5 minutes onto glass slides, followed by staining of the air-dried slides using the May-Grünwald-Giemsa method. Each case was evaluated by a cytopathologist who was unaware of the results of the FCM analysis and centrally reviewed by one of the authors (L.G.). Samples were considered “negative” when contained benign-appearing lymphocytes and monocytes and as “positive” when the cell exhibited atypical cellular features, such as a blastic-like appearance with an increased nuclear-cytoplasmic ratio and/or prominent nucleoli.
FCM analysis
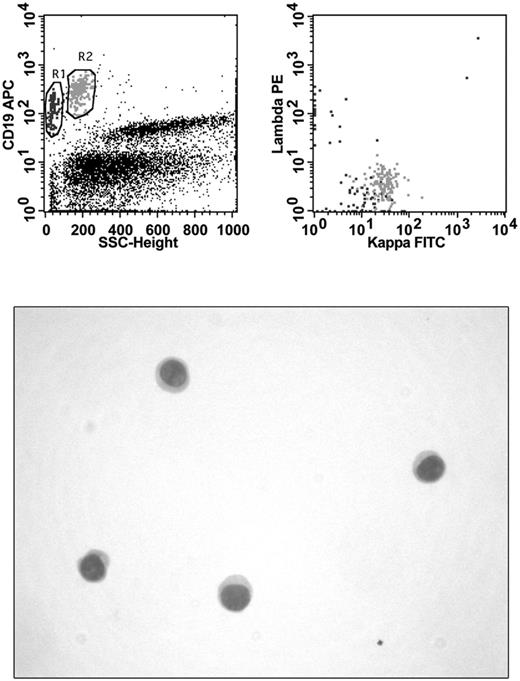
CSF was collected by lumbar puncture in absence of stabilizing medium and sent for laboratory investigations within 1 hour after collection. FCM analysis was performed immediately on receipt in laboratory. CSF volumes for FCM analysis ranged from 0.5-2.0 mL. Specimens were washed with PBS to remove cytophilic Abs and resuspended in 200 μL of PBS. Cellularity and vitality were determined manually by Trypan blue uptake using 10 μL of concentrated cells. Abs in use in the different laboratories involved in the study were added to the cells according to the manufacturer's recommendations. Specimens were stained for 15 minutes at room temperature with mAb combinations selected on the basis of: (1) the quantity of cells observed at manual count, (2) the availability of the 3- or 4-color instrument for FCM analysis, and (3) the patient's clinical history. In cases of low cell count, laboratories that used a 3-color instrument always performed a “minimal panel” (Table 1) consisting of surface light chain determination by 2 tubes. Laboratories that used a 4 color instrument performed the minimal panel in 1 tube. After a washing step, cells were resuspended in 300 μL of PBS and immediately acquired for FCM analysis. Multiparameter analysis was based on antigen expression and morphologic properties were defined by forward and side scatter and performed with a FACSCalibur (BD Biosciences) flow cytometer with software normally in use in each laboratory. B-cell malignancy was diagnosed in the presence of an abnormal pattern of antigen expression and light chain restriction. A sample was considered as positive by FCM when a homogeneous cluster of at least 20 events with immunophenotypic features of neoplastic lymphocytes was detected (Figure 1). All FCM data were centrally reviewed by one of the authors (A.S.) who was unaware of the cytologic diagnosis.
Immunophenotyping and CC in a patient with DLBCL. FCM analysis shows the presence of 2 B-cell populations: one (black) of small polyclonal B lymphocytes and another (gray) of large monoclonal (κ-light chain positive) neoplastic B cells. No evidence of malignancy can be seen in the hypocellular cytospin preparation.
Immunophenotyping and CC in a patient with DLBCL. FCM analysis shows the presence of 2 B-cell populations: one (black) of small polyclonal B lymphocytes and another (gray) of large monoclonal (κ-light chain positive) neoplastic B cells. No evidence of malignancy can be seen in the hypocellular cytospin preparation.
Statistical analysis
The incidence of positive tests for occult CNS dissemination was calculated for each method (FCM and CC) and compared using the McNemar test for paired data. On the basis of test results, patients were classified into 4 groups deriving from the concordance or discordance of tests: both positive (FCM+/CC+), both negative (FCM−/CC−), FCM positive and CC negative (FCM+/CC−), and FCM negative and CC positive (FCM−/CC+). Patient characteristics associated with an FCM+ result were evaluated using logistical regression models, including in the final model the IPI score and all the variables with P < .05 in the univariate analyses. The PFS of patients was defined as the time from the date of lymphoma diagnosis to the date of progression (ie, systemic, brain parenchymal, or meningeal), death from any cause, or the date of the last follow-up visit. OS was calculated from the date of lymphoma diagnosis to the date of death from any cause or the date of the last follow-up visit. Differences in PFS and OS between groups were evaluated using the log-rank test and the Cox proportional hazards model, also adjusting for IPI score, methotrexate treatment, cytarabine treatment, and variables with P < .10 in the univariate analyses of PFS. Investigated variables, in addition to IPI score, were: lymphoma category, HIV infection, B symptoms, bulky disease, BM involvement, and testicle involvement. Cumulative incidence of CNS progression was calculated from the date of lymphoma diagnosis to the date of CNS progression, considering death from any cause as a competing event according to the method of Gooley et al.20 Differences between groups were assessed with the Gray test21 and the Fine and Gray proportional-hazard model.22
Results
Patient characteristics
The characteristics of the 174 patients are listed in Table 2. Multiparameter FCM analysis detected a clonal population in 18 of 174 patients (10%), whereas CC detected abnormal cells only in 7 patients (4%; P < .001). Therefore, procedure results were concordant (FCM+/CC+) in 7 (4%) cases and discordant (FCM+/CC−) in 11 (6%) cases. There was no FCM−/CC+ case. The characteristics of the 18 FCM+ patients were: male sex, n = 12 (66%); median age, 49 years (Interquartile range [IQR]: 36-56); 11 patients had DLBCL (61%), 6 (33%) BL, and 1 (5%) B-LL; and 4 patients (22%) were HIV+. All FCM+ patients had an age-adjusted/IPI score > 1 and 15 (83%) had elevated serum LDH. Extranodal disease sites involved were: BM in 10 (55%), testis in 2 (11%), palate in 1 (5%), and paravertebral masses involvement in 3 (16%) patients. Seventeen patients (94%) had an Ann Arbor stage III-IV and 8 patients (44%) had bulky disease. Treatment in the 18 FCM+ patients consisted of: R-CHOP–like regimen in 11 DLBCL patients, 5 of whom received consolidation with high-dose chemotherapy supported by autologous stem cell transplantation (ASCT); treatment protocols with high-dose methotrexate and high-dose cytarabine (R-HDS or R-CODOX-IVAC) in 7 BL, B-LL, and HIV+ patients.
FCM positivity was significantly associated with the number of WBCs in the CSF (odds ratio = 7.2; 95% CI, 2.2-23.4) and BM involvement (odds ratio = 2.9, 95% CI, 0.9-8.9; Table 3).
Outcome
At a median follow-up of 46 months, 74 patients (42%) experienced failure (progressive disease or relapse): 64 patients had systemic progression and 10 patients (14%) had CNS relapses. Involved CNS areas were: brain parenchyma in 5 DLBCL patients, meninges in 2 (1 B-LL and 1 BL patient), and meninges and brain parenchyma in 1 DLBCL patient; 2 other DLBCL patients had a concurrent brain parenchyma and lymph nodal relapse. Of the 18 FCM+ patients, 13 experienced disease failure: systemic progression in 9 (8 DLBCL and 1 BL patient), CNS relapses in 3 patients (brain parenchymal failure in 2 DLBCL patients and meningeal relapse in 1 BL patient) and both systemic (lymph nodes) and CNS (brain parenchyma) relapse in 1 DLBCL patient. Of the 13 FCM+ patients who experienced relapse, 11 were treated with the R-CHOP–like regimen and 2 were treated with the CODOX-IVAC regimen. Sixty-eight patients died, 54 because of progression of lymphoma, 7 of treatment-related toxicities, and 7 from other causes.
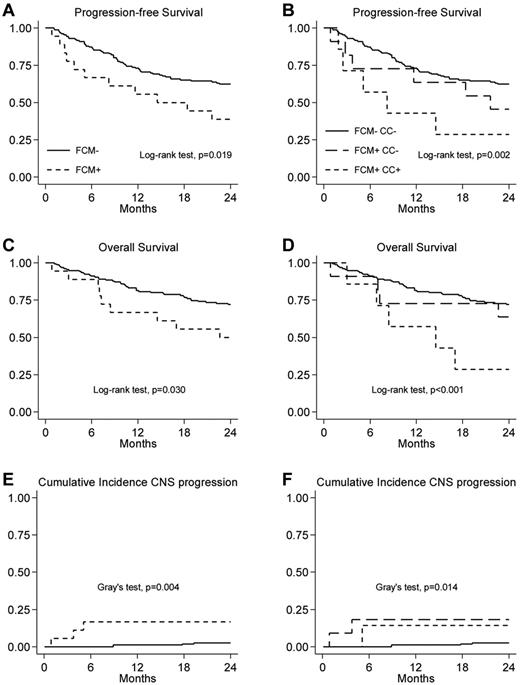
Two-year PFS was 60% (95% CI, 52-67) in the whole group of patients, with a median time to progression of 10 months (IQR, 5-19). The 2-year PFS rate was significantly higher in FCM− patients compared with FCM+ patients: 62% (95% CI, 54-70) versus 39% (95% CI, 17-60; P = .019; Figure 2A), with an adjusted hazard ratio (aHR) of 1.8 (95% CI, 0.9-3.5; Table 4). When we analyzed patients subdivided into 3 groups, FCM−/CC−, FCM+/CC−, and FCM+/CC+, the 2-year PFS rates were 62% (95% CI, 54-70), 45% (95% CI, 17-71), and 29% (95% CI, 4-61), respectively (P = .002; Figure 2B). The progression risk was significantly higher in patients with FCM+/CC+ compared with patients with both negative results in FCM and CC (aHR = 3.1; 95% CI, 1.3-7.5). There was no difference in progression risk in patients with discordant results in FCM and CC with respect to patients both negative in FCM and CC (aHR = 1.2; 95% CI, 0.5-2.9; Table 4).
The 2-year OS was 70% (95% CI, 62-76) for the whole series. The OS was significantly higher in patients who were FCM− compared with those who were FCM+, with a 2-year OS of 72% (95% CI, 64-78) and 50% (95% CI, 26-70), respectively (P = .03; Figure 2C), with an aHR of 6 (95% CI, 0.8-3.2; Table 4). When we analyzed patients subdivided into 3 subgroups, FCM−/CC−, FCM+/CC−, and FCM+/CC+, the 2-year OS was 72% (95% CI, 64-78), 64% (95% CI, 30-85), and 29% (95% CI, 4-61), respectively (P = .001; Figure 2D). The risk of mortality was significantly higher in FCM+/CC+ patients compared with FCM−/CC− patients (aHR = 3.5, 95% CI, 1.4-8.6), whereas the risk of death was not significantly different in patients with discordant FCM/CC results compared with patients with negative results in both FCM and CC (aHR = 10.9; 95% CI, 0.4-2.4; Table 4).
CNS failure risk
The 2-year cumulative incidence of CNS progression was 4% (95% CI, 1-7) in the whole group of patients, with a median time to progression of 9 months (IQR, 4-18). The cumulative incidence of CNS progression was significantly higher in patients FCM+ compared with those FCM−, with a 2-year CNS cumulative incidence of 17% (95% CI, 0-34) versus 3% (95% CI, 0-7), respectively (P = .004; Figure 2E), with an HR of 7.3 (95% CI, 1.6-32.6; Table 4). When we analyzed patients subdivided into 3 subgroups, FCM−/CC−, FCM+/CC−, and FCM+/CC+, the 2-year cumulative incidence of CNS progression were as follows: 3% (95% CI, 0-7), 18% (95% CI, 0-42), and 14% (95% CI, 0-43; P = .021; Figure 2F). The risk of CNS progression was significantly higher both in FCM+/CC− patients and FCM+/CC+ patients compared with FCM−/CC− patients (HR = 8.2; 95% CI, 1.45-46 and HR = 6.0, 95% CI, 0.68-32.6, respectively Table 4).
Discussion
This multicenter, prospective trial showed that CSF involvement confirmed by FCM is a predictor of CNS dissemination and poor outcome in patients with aggressive B-cell lymphoma, which is valuable information that will help clinicians in distinguishing the best candidates to receive CNS prophylaxis or treatment. The present study also showed that the probability of detection of CSF involvement using FCM is higher than with CC alone. The 18 FCM+ samples were easily identified using a single tube with the 4-color “minimal panel.” We showed that FCM had a probability of 10% (compared with 4% with CC) of detecting CSF involvement, with 6% of patients with discordant features (FCM+/CC−). These figures are similar to those previously reported in retrospective studies.7–14 Among others, Hegde et al have shown CSF involvement by FCM in 11 (22%) of 51 B-NHL patients analyzed,12 whereas CC was only able to detect malignant cells in 1 of these 11 patients. More recently, Quijano et al showed the presence of neoplastic B cells by FCM in 27 (22%) of 123 aggressive B-NHL patients,13 whereas CC was either positive or suspicious in only 7 (6%) and 3 (2%) of these patients, respectively. In a recently published paper, Stacchini et al found CNS involvement in 24% of 62 B-NHL samples analyzed, whereas CC gave only 16% positive results.14 The lower rate of FCM+ cases observed in our study (10%) was probably because of the exclusion of patients with signs of neurologic disease, which were included in the previous studies.12–15,23–27
In our study, multivariate analyses showed that the detection of 5 or more WBCs/μL in the CSF samples was a predictive factor of a positive FCM test. Moreover, the proportion of patients with BM involvement was higher in FCM+ patients, suggesting a baseline characteristic that might represent a patient group at increased risk of CNS relapse, as was reported previously.28–32
With a limited number of positive cases, the most relevant contribution of our trial was that FCM+ patients have a higher risk of CNS progression than FCM− patients. FCM+ patients who experienced relapse were mainly DLBCL patients who were treated with conventional chemoimmunotherapy, including high-dose chemotherapy supported by ASCT. None of these patients was treated with CNS-penetrating drugs as high-dose methotrexate and high-dose cytarabine. To test the relationship between the outcomes of systemic treatment and FCM CSF results, we made a multivariate analysis with Cox proportional hazards model, also adjusting for high-dose methotrexate and cytarabine treatment. The analysis showed an increased aHR of 7.3 for FCM+ patients. This result was irrespective of the type of systemic treatment given. However, we could not properly address the prophylaxis advantage of high-dose cytarabine or methotrexate because the choice of systemic treatment was based only on lymphoma histologic subtype and not on FCM CSF results. Moreover, patients with FCM+/CC+ CSF samples had a significantly poorer PFS compared with those FCM−/CC−, and patients with FCM+ CSF samples had a significantly worse OS.
Prospective evaluation of FCM analysis on long-term clinical outcome has been reported in only 2 previous studies. Among the 11 newly diagnosed FCM+ patients with evidence of occult cerebrospinal fluid lymphoma by FCM in Hedge et al, 9 received an active treatment, 1 received prophylactic treatment, and 1 sought treatment at another institution.12 Of these patients, 5 (45%) had CNS relapse and died despite active treatment. All 40 FCM− patients received prophylactic treatment and 3 of 40 cases (8%) had CNS relapse. More recently, Sancho et al prospectively analyzed CSF samples from 105 patients with newly diagnosed aggressive lymphomas, and the results were correlated with the cumulative incidence of CNS relapse and with OS.33 Patients with occult CNS involvement were actively treated in both series by IT therapy, which did not prevent a high rate of CNS relapse or progression. These outcomes further indicate that IT therapy alone is suboptimal for preventing CNS relapse. Our present results and the results of previous studies demonstrate that occult CNS involvement at diagnosis in patients with aggressive NHL is associated with a higher probability of CNS relapse, which requires CNS-tailored treatments and risk stratification regarding CNS prophylaxis. In the absence of identified molecular markers or well-defined clinical parameters that are correlated with CNS invasion, the currently recognized risk factors for CNS relapse may be based on FCM status of the CSF.
In the present study, a significantly lower rate of CNS relapse was observed in patients who were FCM−, but even in this category, relapsed patients were mainly DLBCL. CNS relapse has also been observed in retrospective series of patients identified as belonging to a low-risk group,34,35 leading to the question of whether FCM analysis might also be helpful in these patients. Currently, there is no indication that low-risk patients may benefit from IT chemoprophylaxis. This needs further evaluation that would lead to an improved understanding of the special characteristics related to this subgroup of lymphoma patients.
An important open question raised by our study and others is the need for a more intensive therapy, including drugs with good CNS bioavailability, in lymphoma patients with FCM+ in CSF samples. The best strategy to prevent CNS relapse is still a matter of debate. The addition of rituximab to CHOP chemotherapy (ie, R-CHOP) improves outcome, preventing relapses in patients with aggressive lymphoma. Available literature provides discordant results on the role of rituximab in preventing CNS dissemination.36–39 It is likely that rituximab contributes to a better systemic disease control in DLBCL patients, resulting in a reduced risk of CNS recurrence. However, the results of the present study show that although all patients were treated with rituximab, the incidence of CNS recurrence remained similar (10 of 174; 5.7%) to rates recorded in studies from the pre-rituximab era (5%),1 suggesting that patients who are FCM+ may need an intensification therapy other than the addition of rituximab.
Because most CNS relapses involve the brain parenchyma, the combination of high doses of IV drugs with a good CNS bioavailability, such as methotrexate and cytarabine and IT chemotherapy with methotrexate, free or liposomal cytarabine,40–41 and/or steroids, appears advisable to prevent CNS relapses in lymphoma patients with FCM+ CSF. However, regimens containing high-dose chemotherapy such as methotrexate or cytarabine may not be a reasonable option for a large portion of high-risk older patients with DLBCL because of toxicity.
In conclusion, this prospective trial shows that FCM is more sensitive than CC for the detection of occult leptomeningeal disease in patients with aggressive lymphoma and increased risk of CNS dissemination. FCM+ CSF is an adverse prognostic factor for survival and is associated with a significantly high risk of CNS progression. Different treatment strategies including intensification of the systemic treatment and/or more effective IT treatments therapies to prevent CNS recurrence should be explored in future studies in a larger series of FCM+ patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients, nurses, physicians, and clinical trial office staff (Giorgio Priolo, Pasqualina De Masi, and Francesca Pirillo) for their help with this study and Gianni Ciccone from CPO Piemonte.
This work was supported by an unrestricted grant from the Ministero della Salute, Dipartimento dell'Innovazione-Direzione Generale Ricerca Scientifica e Tecnologica (Progetto di Ricerca Finalizzata Bando 2008, u.p.b. 3.1.2.10, scientific research, chapter 3398).
Authorship
Contribution: G.B., U.V., and E.M.P. designed the study; G.B. and U.V. supervised the study, analyzed the data, and wrote the manuscript; A.E. analyzed and interpreted the data; M.S., A.J.M.F., U.V., and E.M.P. reviewed and commented on the manuscript; and all authors enrolled patients and reviewed and approved the final manuscript.
Conflict-of-interest disclosure: U.V. was on the advisory committee of Roche Italy and received a lecture fee from Roche. The remaining authors declare no competing financial interests.
Correspondence: Umberto Vitolo, MD, S.C. Ematologia 2, Azienda Ospedaliera Città della Salute e della Scienza, Corso Bramante 88 10126 Torino, Italy; e-mail: uvitolo@molinette.piemonte.it.