Abstract
Telomere attrition induces cell senescence and apoptosis. We hypothesized that age-adjusted pretransplantation telomere length might predict treatment-related mortality (TRM) after hematopoietic stem cell transplantation (HSCT). Between 2000 and 2005, 178 consecutive patients underwent HSCT from HLA-identical sibling donors after myeloablative conditioning regimens, mainly for hematologic malignancies (n = 153). Blood lymphocytes' telomere length was measured by real-time quantitative PCR before HSCT. Age-adjusted pretransplantation telomere lengths were analyzed for correlation with clinical outcomes. After age adjustment, patients' telomere-length distribution was similar among all 4 quartiles except for disease stage. There was no correlation between telomere length and engraftment, GVHD, or relapse. The overall survival was 62% at 5 years (95% confidence interval [CI], 54-70). After a median follow-up of 51 months (range, 1-121 months), 43 patients died because of TRM. The TRM rate inversely correlated with telomere length. TRM in patients in the first (lowest telomere length) quartile was significantly higher than in patients with longer telomeres (P = .017). In multivariate analysis, recipients' age (hazard ratio, 1.1; 95% CI, .0-1.1; P = .0001) and age-adjusted telomere length (hazard ratio, 0.4; 95% CI; 0.2-0.8; P = .01) were independently associated with TRM. In conclusion, age-adjusted recipients' telomere length is an independent biologic marker of TRM after HSCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative procedure for many malignant and nonmalignant hematologic blood disorders. However, transplantation remains associated with a significant mortality rate because of relapse and nonrelapse causes. Outcomes of HSCT have improved over time, but treatment-related mortality (TRM) remains a major concern. TRM is mainly associated with acute and chronic graft-versus-host disease (GVHD), infections, interstitial pneumonia, and toxicities of the HSCT procedure.1 Estimates of overall survival (OS) and of TRM are of primary clinical importance in weighing the risk/benefit ratio of allogeneic HSCT. Standard factors, such as age, type, and stage of the underlying disease, as well as pretransplantation comorbidities are not sufficient to precisely predict transplantation tolerance and posttransplantation survival.2 Some biomarkers, such as elevated pretransplantation serum ferritin and C-reactive protein (CRP) levels, have been suggested to be associated with an increased risk of morbidity and mortality after HSCT.3–5
Telomeres are highly conserved protective terminal chromosomal structures consisting of hundreds to thousands of tandem TTAGGG hexamers and their associated shelterin proteins. Telomeres shorten with every mitotic cell division, and telomere attrition has a fundamental role in cell senescence. The recent discovery that inherited mutations in genes that encode for proteins that repair or protect telomeres are etiologic in a range of human diseases has disclosed clinical manifestations in diverse tissues, including the bone marrow, lung, and liver, indicating that defects in telomere repair and protection can cause organ failure.6 Dyskeratosis congenita (DC), an inherited type of aplastic anemia, is caused by mutations in the telomerase complex genes. HSCT is the only curative treatment for bone marrow failure in patients with DC, but case reports and small series of patients have suggested substantial risks of toxicity from radiation and chemotherapy, as well as immune-related complications such as infections. As a result, long-term survival of patients with DC after HSCT has been poor because of TRM, including graft failure, GVHD, sepsis, pulmonary fibrosis, cirrhosis, and veno-occlusive diseases.7,8
HSCT has been considered an experimental opportunity to observe telomere dynamics in a highly proliferative cell environment. The telomere length of marrow cells from HSCT recipients was found considerably shorter than telomere length of donor cells, likely reflecting an immediate increased replicative demand after transplantation.9–11 That telomere attrition might have a role in or serve as a marker of long-term outcome after HSCT has been an attractive hypothesis, but data are limited (for a review, see Gadalla et al12 ). The potential impact of pretransplantation telomere length on HSCT outcomes, notably TRM, has not been reported.
We hypothesized that age-adjusted pretransplantation telomere length might predict TRM after HSCT. To determine the effects of telomere attrition on HSCT results, we measured the pretreatment telomere length in 178 consecutive patients who had undergone myeloablative HSCT from a matched related donor, and we analyzed for correlations with outcomes after HSCT.
Methods
Patients' characteristics
All consecutive patients who underwent an HSCT after a standard conditioning regimen13 at Saint Louis Hospital between January 2000 and December 2005 and who received transplants from an HLA-identical sibling donor were included in the study. GVHD prophylaxis consisted of cyclosporine and methotrexate for all patients. Antithymocyte globulin was administered to patients with nonmalignant diseases. A total of 178 patients from whom sufficient samples were available for testing were included in the analysis. Patients (or legal guardians) signed informed consent according to approved protocols by the Institutional Review Board of the Hospital Saint Louis. This study was conducted in accordance with the Declaration of Helsinki. Patient and disease characteristics are summarized in Table 1.
End point definitions
Neutrophil recovery was defined as the first of 3 consecutive days with a neutrophil count of at least 0.5 × 109/L, and platelet recovery as the first of 7 consecutive days with an unsupported platelet count of at least 20 × 109/L. Acute and chronic GVHD were diagnosed and graded according to published criteria,14 with histopathologic confirmation when possible. Disease risk was assessed according to the recent European Leukemia Network classification15 concerning acute myeloid leukemia (AML) in first complete remission. For acute lymphoid leukemia (ALL), only patients with high-risk disease received transplants in first complete remission, according to our local clinical trials policy.16 Disease-stage categories were defined by ranking diseases according to the number of previous (pretransplantation) treatments received. Nonmalignant diseases were classified separately. Early stage diseases included patients with malignant diseases who received transplants in first complete remission as well as patients with chronic myelogenous leukemia in chronic phase. Advanced-stage diseases included malignant diseases transplanted beyond first complete remission as well as patients not in complete remission at time of HSCT. Karnofsky performance status (KPS) was calculated in the 2 weeks before begin the conditioning regimen. Serum ferritin and C-reactive protein (CRP) levels were collected within the month and 2 weeks before the start of the conditioning regimen, respectively.
Telomere-length measurement
Telomere length of pretreatment peripheral blood leukocytes was assessed by quantitative PCR, as previously described,17 but with some modifications. Total leukocytes were separated by ammonium-based lysis of red blood cells, and DNA was extracted using the DNeasy Blood kit (QIAGEN). PCRs were performed in a Rotor Gene-Q (QIAGEN). Each sample was assayed in triplicate and each reaction contained 1.6 ng of genomic DNA, 7.2 pg of each primer (except for single copy gene reverse primer that contained 12 pg), and 16 μL of Rotor-Gene SYBR Green PCR Master Mix (QIAGEN). Each sample's telomere length (x) was based on the telomere to single copy gene ratio (T:S ratio) and based on the calculation of the Ct [Ct(telomeres)/Ct(single gene)]. Telomere length was expressed as the relative T:S ratio, which was normalized to the average T:S ratio of reference sample [2−(Ctx − Ctr) = 2−Ct], used for the standard curve, as reference sample, and as validation sample. To compare results from different plate runs, the results of each plate were approved only if the relative T/S ratio of the validation reference sample fell within 3% variation. Laboratory personnel conducting the telomere-length assay were blinded to patients' clinical outcomes before the statistical analysis.
Statistical methods
Age-adjusted telomere length for each subject was computed by subtracting the subject's linear predicted telomere length from the observed telomere length. Quartiles of the age-adjusted telomere length were used to evaluate the probabilities of engraftment, acute GVHD and cGVHD, TRM, relapse, and overall survival. Summary statistics (means, proportions, and standard deviations) stratified by age-adjusted telomere quartiles were used to describe patients' age, sex, and other baseline characteristics. P values based on multisample tests for proportions and the analysis of variance tests or Kruskall Wallis nonparametric tests were used to compare patients' baseline characteristics. Kaplan-Meier analysis was used to calculate survival rates. P values from the log-rank tests were used to evaluate the overall covariate effects in the univariate analysis of OS. Univariate and multivariate methods developed by Fine and Gray for competing risks studies18 were used for assessment of prognostic factors of TRM with relapse as a competing event. All tests were 2-sided, with type I error rate fixed at 0.05. To improve the opportunity to discover unsuspected correlations, we purposely did not adjust results for multiple comparisons. Statistical analyses were performed with SPSS 19 software, Stata 11, and R packages “cmprsk” (competing risks).
Results
Telomere length in patients before HSCT
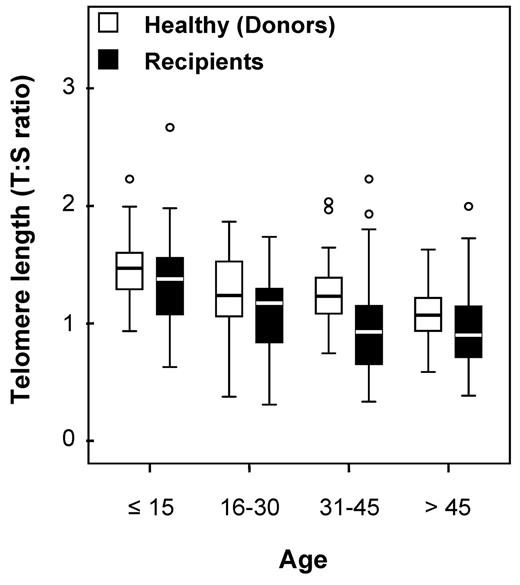
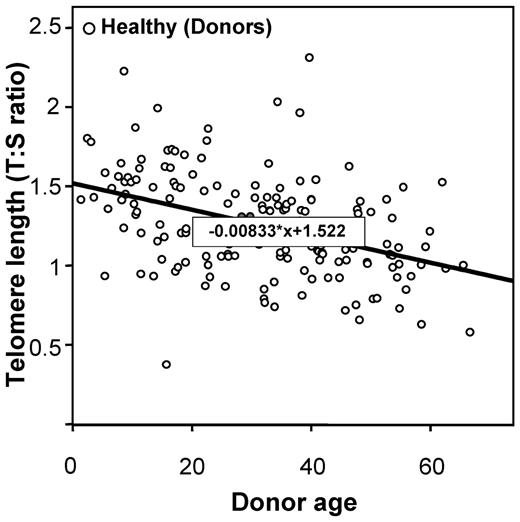
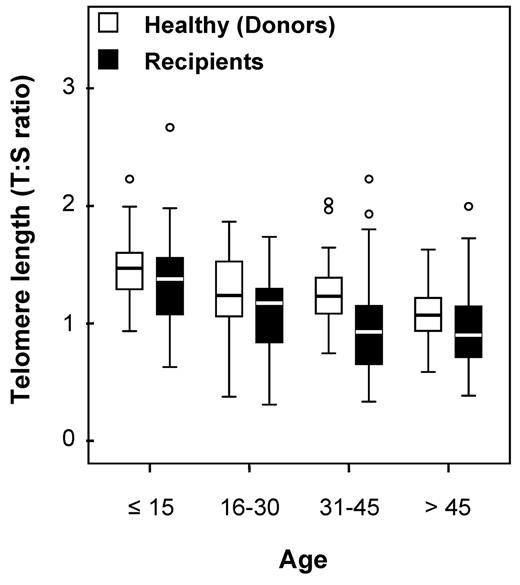
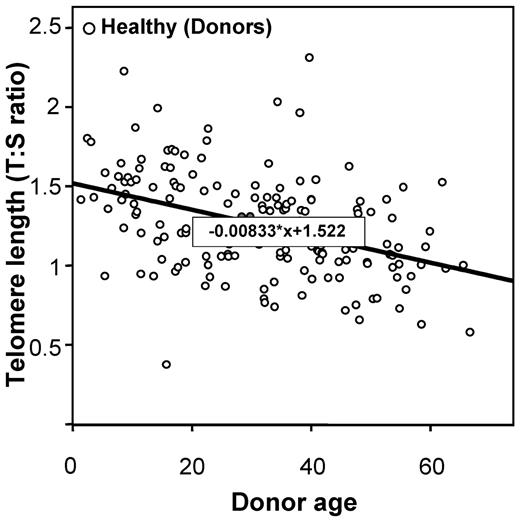
Telomere lengths were measured in 178 recipients and in 173 healthy HLA-identical sibling donors who served as controls (DNA was missing for 5 individuals). Telomere lengths were shorter in patients than in their sibling donors in all tested age brackets (Figure 1). The median age of both recipients and donors was comparable (31.8 [range, 3.4-64.8] and 31.6 years [range, 1.4-66.8], respectively; P = .78). We then determined the age-adjusted pretransplantation telomere length in patients' samples; telomere length was adjusted for age according to the telomere-length attrition rate with aging in the donor control group. Because the distribution of the telomere length was linear from 10 to 60 years of age in controls, we thus determined the linear curve of telomere-length distribution according to age (Figure 2; there were only few patients younger than 10 or older than 60 years of age [n = 17 and 4, respectively]). We categorized the patient population in quartiles based on age-adjusted telomere length (Table 1). Patient characteristics were similar among all 4 quartiles, except for disease stage (with more advanced-stage malignant diseases in patients with shortest telomeres [first quartile]).
Age bracket dot plot representation of telomere-length distribution between donors (left) and recipients (right).
Age bracket dot plot representation of telomere-length distribution between donors (left) and recipients (right).
Linear curve representation of telomere-length distribution according to the age.
Linear curve representation of telomere-length distribution according to the age.
Telomere length and outcomes after HSCT in the total population
The median follow-up of the study was 51 months (range, 1-121 months). All patients engrafted. The median time to achieve absolute neutrophil count > 500/μL was 18 days (range, 4-45) and median time to platelet count > 20 000/μL was 17 days (range, 7-58). Cumulative incidence of acute GVHD grade II-IV was 45% (95% confidence interval (CI), 37-53) and chronic GVHD was 41% at 36 months (95% CI, 33-49). Thirty-four patients relapsed, leading to a cumulative incidence of 22% at 5 years (95CI 16%-28%). There was no correlation between telomere length and engraftment, acute or chronic GVHD, or relapse (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
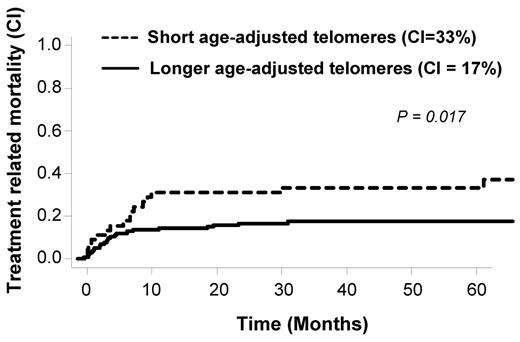
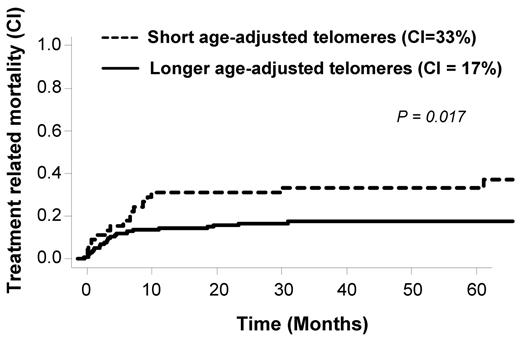
We have previously observed in patients with severe aplastic anemia treated with immunosuppression a worse clinical outcome in patients in the lowest quartile of telomere length.19 Here, we adopted the same strategy for OS and TRM analysis. We separated patients into 2 groups: shorter telomeres (first quartile) and longer telomeres (upper 3 quartiles). The OS was 51% at 5 years in the shorter (first) quartile in comparison to 65% in the longer quartiles (2, 3, and 4; P = .07). During the study, 43 patients died because of TRM. Causes of death are detailed in Table 2. As shown in Figure 3, TRM rate inversely correlated with telomere length. Patients with shorter telomeres showed a statistically significant increased TRM (P = .017). In multivariate analysis using competing risk regression (including age-adjusted telomeres length, disease stage, age, total body irradiation (TBI), and source of stem cells), age of the recipients (HR, 1.1, 95% CI [1.0-1.1, P = .0001]) and age-adjusted telomeres (HR, 0.4, 95% CI [0.2-0.8, P = .01]) were independently associated with TRM (Table 3). KPS was not included in the model because the majority of our patients were scored ≥ 90% (148 of 161 with available KPS before transplantation). In addition, pretransplantation serum ferritin and CRP levels were not associated with TRM in our population (supplemental Figures 1 and 2, respectively) and thus not considered for multivariate analysis. We also tested the potential role of donor telomere length but did not found any correlation with outcomes post-HSCT (Table 4).
Telomere-length and treatment-related mortality cumulative incidences in the overall population.
Telomere-length and treatment-related mortality cumulative incidences in the overall population.
Telomere length and outcomes after HSCT in patients with malignant diseases
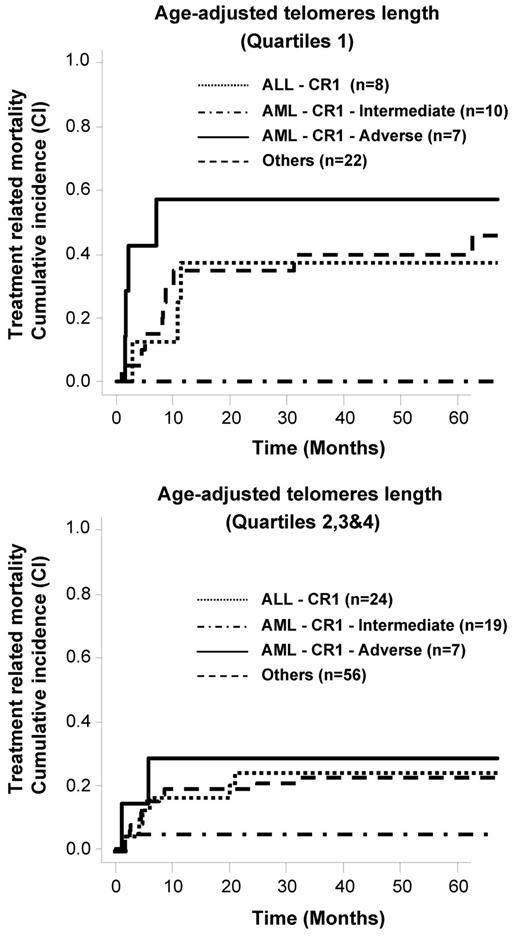
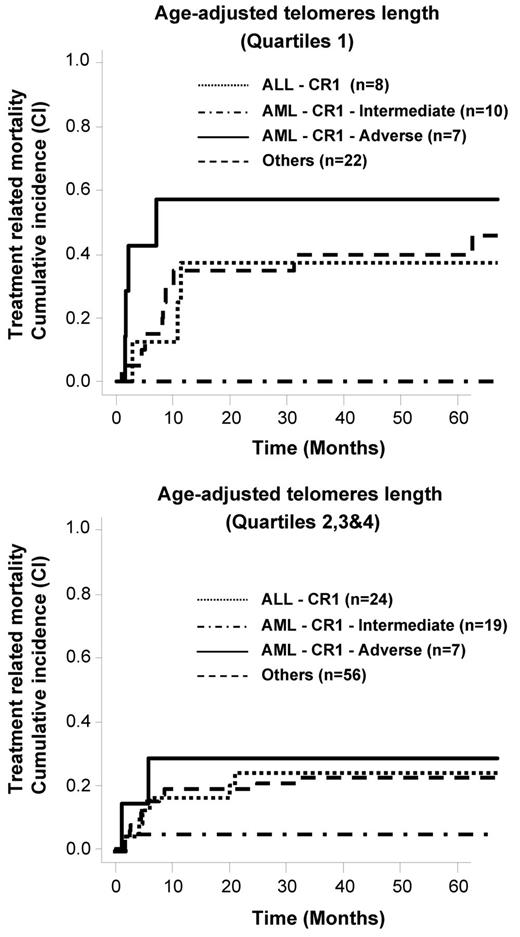
As patients with nonmalignant diseases usually have a better outcome in comparison to patients with malignant diseases, we performed an analysis restricted to the 153 patients with malignant disorders. Again, a higher rate of TRM was observed for patients who had shorter telomeres, especially for patients with advanced-stage disease (supplemental Figure 3). In multivariate analysis, the same 2 independent factors (age and age-adjusted telomere length) were present when analysis was restricted to the patients in the malignant disease group (n = 153; respectively, P = .0004, HR 1.1 and P = .018, HR 0.43). We also included the disease risk as stated in Table 1 in the model (AML CR1 intermediate risk, AML CR1 adverse risk, ALL CR1 and other diseases). Age and age-adjusted telomere length remained the only 2 factors associated with TRM (respectively, P = .001, HR 1.1 and P = .018, HR 0.42). As shown in Figure 4, telomere length predicted TRM in all disease-risk subgroups except for intermediate-risk AML where the TRM was low.
Telomere-length and TRM cumulative incidences according to the disease risk in patients with malignant diseases.
Telomere-length and TRM cumulative incidences according to the disease risk in patients with malignant diseases.
Discussion
To our knowledge, this is the first study to evaluate the impact of leukocyte pretransplantation telomere length on TRM in the context of HSCT. Accelerated telomere shortening has been reported to occur after allogeneic HSCT.10,11,20 Telomere attrition has mainly been observed during the first year after allogeneic HSCT; thereafter, telomere-length dynamics of recipients appear not to differ from those of their donors.21–23 Chronic GVHD and a female donor were associated with greater differences in telomere length between donor and recipient after HSCT.24 Here, we found that shorter leukocyte pretransplantation telomere length correlated with higher TRM posttransplantation.
Genetic predisposition (telomerase gene mutations), environmental factors (reactive oxidative stress), and iatrogenic interventions (chemotoxic drugs and radiation) all may accelerate telomere loss. In our patients, in the shortest quartile of telomere length, there may be unidentified genetic lesions relating to telomere repair or structure, or they may have inherited shorter telomeres from their parents. However, genetic predisposition is reliably predicted by extremely short telomere length, and our patients' values were within the normal range. The influence of chemotherapy on telomere length has been demonstrated in breast cancer patients after multiple chemotherapy courses25 and in lymphoma patients undergoing high-dose chemotherapy courses.26 Accelerated telomere shortening has also been observed after autologous HSCT and appears to persist.27–29 Telomere shortening may be related to pretransplantation exposure to multiple courses of chemotherapy in our population, as suggested by the association of advanced-stage disease and shorter telomere length. It seems unlikely that telomere length could have been influenced by circulating blast cells because no patients with refractory acute leukemia were included and patients with other refractory diseases need to be sensitive to chemotherapy before HSCT with usually no malignant circulating cells at time of transplantation. Nevertheless, in the current study, short telomere length was a survival risk factor independent of disease stage, the latter including by definition the number of previous chemotherapy cycles (first or second remission).
As accelerated telomere loss postautologous HSCT has been implicated as a predictor for the development of therapy-related myelodysplasia and acute myeloid leukemia,30 this independent association between short telomeres and TRM may be pathophysiologic, not simply a biomarker. Patients rated as advanced stage by disease, prior relapses, and remission, or who have equivalent records of chemotherapy or radiation may not have equivalent functional consequences to these exposures, for reasons ranging from variations in medical practice to differences in drug metabolism pathways. Cumulative damage to DNA may be better reflected by telomere length than by simple medical history or complex genomic analysis. Causes of death were equally distributed in the first quartile compared with the other 3 quartiles, consistent with a nonorgan specific but general phenomenon. We observed the same number of deaths (n = 4) from multiorgan failure in the first quartiles in comparison with the other 3 quartiles, in favor of this hypothesis. Telomere length could thus reflect accelerated “physiologic” aging in our population. A previous study evaluated the cost, in terms of telomere loss, of delivering high-dose cytarabine corresponded to that normally paid in approximately 20 years of life in the absence of nonphysiologic hematopoietic stress.26 In our population, we found shorter telomere lengths in recipients in all tested age brackets, especially after 30 years old, more or less the age limit where the intensity of the conditioning regimen (reduced vs standard) is regularly discussed. However, recipients' hematopoietic cells were chosen for analysis as a surrogate for telomere length in other affected tissues and may not directly reflect the telomere length in affected organs. Nevertheless, a good correlation of telomere length in leukocytes, buccal mucosa swab, and fibroblasts has been demonstrated,31 suggesting that the telomere length in peripheral blood leukocytes may reflect the length of telomeres in other tissues.
A conventional high-dose transplantation preparative regimen causes serious toxicity to organs such as the gut, liver, kidneys, heart, and lungs.2 Reduced-intensity or nonmyeloablative conditioning regimens have relied on graft-versus-tumor effects for disease control, and their advent has allowed relatively older and more severely ill patients to be offered allogeneic HSCT. It has become increasingly important to optimize pretransplantation risk assessment to improve HSCT decision-making and clinical trial assignments. Chronologic age has been long used as the main HSCT prognostic variable as exclusion criteria for high-dose conditioning regimen, but age alone is a poor predictor of HSCT outcome32 ; in some studies there has been little impact of chronologic age on allogeneic HSCT outcomes.33,34 Recent efforts have resulted in the advent of a weighted scoring system that could sensitively capture multiple-organ comorbidities before HCT. Those studies assess not only biologic aging composed of chronologic age but also physical function and organ comorbidities (for a review, see Sorror et al2 ). However, our population was mainly composed of young patients with no comorbidities and in good physical status (KPS ≥ 90%) for which previously described biomarkers (pretransplantation serum ferritin and CRP levels) were not useful. If our results are independently confirmed, pretransplantation telomere length might be a new biomarker helpful for physicians to choose among treatment options, as, for example, the choice of conditioning regimen. Pretransplantation telomere length is thus able to predict TRM in a population where the common predictors (except chronologic age, also found as an independent-associated factor) are not helpful and might identify patients more likely to benefit from reduced-intensity conditioning.
Recipient pretransplantation telomere length was not associated either with engraftment or GVHD. Telomere length is a biologic marker of replicative history and predicts remaining proliferative capacity in vitro.35 Extensive telomere shortening is reported to be involved in late graft failure post-HSCT.36 None of our patients experienced engraftment failure. Graft failure is mainly due to immunization pre-HSCT or an unrelated source of stem cells (notably umbilical cord blood), circumstances that were excluded from the current work. Telomere length did not appear to be critical for hematopoietic recovery, perhaps explained in part by the clinical transplantation setting, as only matched related sibling transplantations were evaluated. In our population of patients receiving HLA-identical grafts, genetic differences in minor histocompatibility antigens triggered GVHD. Donor T cells are responsible for GVHD and recognized minor HA initially presented by recipient-derived dendritic cells.37 Previous experimental studies have demonstrated that increased risk of GVHD in older patients is linked to older dendritic cells. We also did not find any correlation between donor telomere length and outcomes post-HSCT. Our donor population is relatively homogeneous, mainly young healthy subjects who are HLA-identical to recipient patients, and it was not unexpected that donor telomere length would not be predictive of outcomes.
Our work has strengths and limitations. The strengths include the homogeneous cohort prospectively enrolled in our center over a short period of time, and specified diagnostic criteria, homogeneous conditioning regimen, GVHD prophylaxis, supportive care, and prospectively determined and defined clinical outcomes. We monitor our patients indefinitely at specified intervals, and our policy requires periodic assessment for adverse events like relapse and late toxicity. Our study is limited because of the retrospective nature of the analysis and the relatively small number of patients, which did not allow for validation testing in a separate cohort or for reliable subgroup analysis. As a research facility and referral center, our patient population also may not be representative and our results not necessarily generalizable. Therefore, our results need to be replicated to validate the observed associations and to determine reliable telomere-length thresholds that could be incorporated in treatment algorithms.
In conclusion, age-adjusted recipient pretransplantation telomere length was identified as an independent biologic marker of TRM after HSCT from related donors using a myeloablative conditioning regimen. Prediction of important disease-related complications is critical to risk stratification and patient management. Pre-HSCT telomeres length thus appear as a new predictor of TRM that need to be confirmed on independent cohort of patients and on other HSCT setting.
This work was presented orally at the annual meeting of the American Society of Hematology, December 12, 2011, San Diego, CA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health Intramural Research Program, National Heart, Lung, and Blood Institute. R.P.d.L. received a bursary award from the AA and Myelodysplastic Syndrome International Foundation and a grant from France HPN. R.T.C. was supported in part by Fapesp.
Authorship
Contribution: R.P.d.L., R.T.C., G.S., and N.S.Y. conceived and designed the study; R.P.d.L., R.T.C., M.B., J.A., N.A., M.R., J.L., N.D., A.X., D.C., A.T., P.L., G.S., and N.S.Y. provided study materials and patients; R.P.d.L., R.T.C., M.B., J.A., N.A., and P.L. collected and assembled data; R.P.d.L., R.T.C., M.B., G.S., and N.S.Y. analyzed and interpreted data; R.P.d.L., R.T.C., M.B., G.S., and N.S.Y. wrote the manuscript; R.P.d.L., R.T.C., M.B., J.A., M.R., J.L., N.D., A.X., E.C., D.C., A.T., P.L., G.S., and N.S.Y. gave final approval of the manuscript; and R.P.d.L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Régis Peffault de Latour, Service d'Hématologie Greffe, Hôpital Saint Louis, Paris, France; e-mail: regis.peffaultdelatour@sls.aphp.fr.
References
Author notes
R.P.d.L., R.T.C., and M.B. share the first authorship.
G.S. and N.S.Y. share the last authorship.