Abstract
β2-glycoprotein I (β2GPI) is the major antigenic target for antiphospholipid Abs. Anti-β2GPI Abs are a heterogeneous population of Igs targeting all domains of the molecule. Abs specific to β2GPI domain I are strongly associated with thrombosis and obstetric complications. In the present study, we sought to understand the possible pathogenic mechanism for this subset of anti-β2GPI Abs, investigating their potential cross-reactivity with other self-proteins involved in inflammatory or coagulant events. We compared the amino acid sequence of the β2GPI domain I with human proteins in a protein databank and identified a peptide sharing 88% identity with an epitope of human TLR4. A high percentage of patients with antiphospholipid syndrome (41%) and systemic lupus erythematosus (50%) presented serum IgG specific to this peptide. Anti-β2GPI peptide Abs binding the TLR4 were able to induce NF-κB activation in HEK293 cells that were stably transfected with the TLR4 gene. Anti-β2GPI peptide Abs induced activation of TLR4 and triggered interleukin-1 receptor-associated kinase phosphorylation and NF-κB translocation, promoting VCAM expression on endothelial cells and TNF-α release by monocytes. In conclusion, our observations suggest a novel pathogenic mechanism in the TLR4 stimulation by anti-β2GPI peptide Abs that links adaptive immune responses with innate immunity in antiphospholipid syndrome and systemic lupus erythematosus.
Introduction
Antiphospholipid syndrome (APS) is characterized clinically by vascular thrombosis and/or recurrent pregnancy morbidity and serologically by the presence of serum antiphospholipid Abs (aPLs).1–3 The aPLs recognize phospholipids, phospholipid-binding plasma proteins, and/or plasma protein-phospholipid complexes. Among the involved phospholipid-binding plasma proteins, which include prothrombin,4 protein C,5 and protein S,6 β2-glycoprotein I (β2GPI) is now recognized as the most relevant antigenic target for aPLs.7 Anti-β2GPI Abs are composed of subpopulations that differ in their biologic activity and bind with different epitopes on the molecule.8,9 Indeed, β2GPI-dependent aPLs represent formal diagnostic tools and predictive markers for the clinical manifestations of APS.10
β2GPI, one of the most abundant human plasma proteins, consists of 326 amino acids arranged into 5 short consensus repeats (or domains). The molecule interacts with several coagulation components and with different cell types, anchoring its fifth domain to anionic phospholipids on the cell surface.11,12 Although experimental models showed that anti-β2GPI Abs and β2GPI-dependent aPLs derived from patients with APS promote miscarriage and thrombosis in both the venous and arterial systems,13,14 the precise disease-causing molecular mechanisms triggered by these populations of Abs remain undefined. It has been hypothesized that anti-β2GPI Abs binding the proteins complexed with the coagulation factors or expressed on cell membranes associated with adaptor molecules, such as annexin A2,15 TLR2,16,17 TLR4,18,19 and TLR8,20 might induce different biologic effects that contribute to the whole spectrum of the clinical manifestations of APS. Growing evidence demonstrates that anti-β2GPI Abs are able to activate endothelial cells,21 monocytes,22 and platelets,23 leading these cells to a proinflammatory and procoagulant phenotype and thus increasing the risk of thrombosis. In particular, Abs specific to domain I of β2GPI are found to be associated with thrombosis and pregnancy morbidity.24–28 β2GPI domain I might contain different Ab-binding epitopes involved in the pathogenesis of APS that have not yet been completely identified.
The purpose of the present study was to investigate the hypothesis of an antigenic cross-reactivity between anti-β2GPI domain I Abs and other self-proteins potentially involved in inflammatory or coagulant events. By comparing the amino acid sequence of β2GPI domain I with human sequences in a protein databank, we identified a β2GPI domain I peptide (PDDLPFST) that shares 88% identity with an epitope within the extracellular domain of human TLR4 (PDNLPFST). In this study, we evaluated: (1) the presence of anti-β2GPI peptide Abs in serum from patients with APS and systemic lupus erythematosus (SLE); (2) the cross-reactivity between anti-β2GPI peptide Abs and human TLR4; and (3) the possible pathogenic role of anti-β2GPI peptide Abs in inducing the proinflammatory phenotype in endothelial cells and monocytes.
Our data provide evidence that autoantibodies specific to a peptide of the β2 glycoprotein I, which was detected in serum from patients with APS and SLE, are able to engage human TLR4 directly, inducing a proinflammatory phenotype in endothelial cells and monocytes and providing a novel pathogenic mechanism of TLR4 stimulation in APS and SLE.
Methods
Patients
We studied 71 unselected outpatients attending the Lupus Clinic of “Sapienza” University of Rome: 27 patients with APS (median age of 43 years; range, 27-71), in particular, 11 patients with primary APS, 16 with APS secondary to SLE, and 44 with SLE (median age of 37 years; range, 19-66). APS was diagnosed according to the Sapporo criteria.29 SLE was diagnosed in accordance with the American College of Rheumatology revised criteria.30 All patients were tested for IgG and IgM specific to cardiolipin and β2GPI by ELISA using the QUANTA Lite detection kit (INOVA Diagnostic). As controls, we also enrolled 30 healthy subjects matched for sex and age. Serum samples from 16 patients with moderate chronic periodontitis diagnosed according to the American Academy of Periodontology classification,31 were obtained from the Department of Experimental Medicine of “Sapienza” University of Rome. All of the subjects were evaluated by measuring clinical parameters (ie, periodontal probing pocket depth, bleeding on probing, and oral radiographs). As controls, we enrolled 20 healthy subjects without clinical or radiographic evidence of periodontal disease. Human venous blood samples were obtained after informed consent from patients, and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. The local ethics committee approved the study.
Protein alignment strategy
The amino acid sequence of β2GPI domain I was divided into segments of approximately 15 residues, with 5 residues overlapping that were compared with a nonredundant database (National Center for Biotechnology Information; NCBI) to establish the peptide identities using the BLAST tool. We searched for short, nearly exact matches with an established threshold for the expected value (E value) below 0.02.
Peptides
We used a multiple antigen peptide (MAP) containing 8 copies of the peptide PDDLPFST, synthesized directly on a 8-armed branched lysine core (PDDLPFST MAP-8; Primm Labs) for ELISA. The peptide PDDLPFST and the unrelated scramble peptide DSPLTFDP (Primm Labs) were both used as control peptides for inhibition experiments, and the first one was also used for affinity purification of Abs.
ELISA to detect anti-β2GPI peptide Abs
An indirect ELISA was developed essentially as described previously.32 In brief, flat-bottomed polystyrene plates (Maxisorp; Nunc) were coated with the peptide PDDLPFST MAP-8 (2 μg/well) in PBS, pH 7.4, and incubated overnight. Plates were blocked with PBS-3% milk, and incubated with human sera diluted 1:25 in PBS-1% milk at room temperature. HRP-conjugated goat anti–human IgG (Bio-Rad) and O-phenylenediamine dihydrochloride (Sigma-Aldrich) were used. The optical density (OD) was measured at 490 nm. Means + 2 SDs of the OD readings of the healthy controls were considered the cutoff level for positive reactions. All assays were performed in quadruplicate.
For inhibition analysis, we incubated purified anti-β2GPI peptide Abs (1 μg), prepared as described under “Purification of human Abs,” with human recombinant TLR4 synthesized by the eukaryotic translation apparatus of wheat germ (0.5-2 μg; Abnova) in 100 μL of PBS overnight.
ELISA to detect anti–Porphyromonas gingivalis Abs
The sera levels of IgG Abs specific for the bacterial antigens of P gingivalis in the sonicated extract were determined using an indirect ELISA as described previously.33 The cells of P gingivalis, kindly provided by Dr P. Mastrantonio (Istituto Superiore di Sanità, Rome, Italy), were resuspended in PBS containing a protease inhibitor cocktail diluted 1:1000 (P-8465; Sigma-Aldrich) and subjected to ultrasonic disruption at output 3 in a sonifier (Bronson Sonic Power) for 20 minutes.
For ELISA analysis, the sonicated extract was suspended in 0.05M carbonate buffer (Na2CO3/NaHCO3), pH 9.6, at 10 μg/mL; 200 ng of this suspension was placed in each well of a polystyrene microplate (Maxisorp; Nunc) and incubated overnight at 4°C. The plate was blocked with PBS-3% milk and incubated with human sera diluted 1:50 in PBS-1% milk. HRP-conjugated goat anti–human IgG (Bio-Rad) and O-phenylenediamine dihydrochloride (Sigma-Aldrich) were used.
Purification of human Abs
The synthetic peptide PDDLPFST and BSA (Sigma-Aldrich; 5 mg/g of dried Sepharose powder) were coupled to Sepharose 4B (Pharmacia) according to the manufacturer's instructions. A pool of serum samples from 5 patients with APS and SLE positive for anti-β2GPI peptide Abs were diluted in PBS and applied to the Sepharose-peptide column. The effluent material that did not bind to the affinity column was harvested and used as a control (ie, serum deprived of anti-β2GPI peptide Abs). Bound Abs were eluted with 0.1M glycine, pH 2.5, and dialyzed against PBS. Anti-BSA Abs were purified from a pool of sera from 5 healthy subjects. The purity of the preparations was assessed by 10% SDS-PAGE, followed by silver staining (Bio-Rad). The Ab preparations were treated with Detoxi-Gel Endotoxin Removing Gel (Pierce) and contained < 0.00025 ng of endotoxin/μg of protein, as determined by the Limulus amebocyte lysate test (Associates of Cape Cod). The Ab concentration was determined using Micro BCA Protein Assay kit (Pierce). After purification, Abs were tested by Western blot analysis and ELISA to exclude variation in binding reactivity after the purification process. Affinity-purified anti-β2GPI peptide Abs were used in all experiments.
Cells, culture conditions, and treatments
PBMCs from the venous blood of healthy donors were isolated by Ficoll-Hypaque density-gradient separation (Lympholyte-H; Cedarlane Laboratories). CD14+ monocytes were purified by incubation with anti-CD14–coated microbeads (Miltenyi Biotec), followed by sorting with a magnetic device (MiniMacs Separation Unit; Miltenyi Biotec) according to the manufacturer's instructions. The human monocytic cell line THP1 was grown in RPMI (Invitrogen) containing 10% FBS (Gibco-BRL).
Human umbilical vein endothelial cells (HUVECs; Provitro) were grown in endothelial cell growth medium (Invitrogen) containing 10% FBS (Gibco-BRL).
Human embryonic kidney 293 (HEK293) cells, stably transfected with TLR4 and the coreceptors MD2 and CD14 (InvivoGen) and cotransfected with the pNifty plasmid (HEK293T4), were a kind gift of Prof A. Puccetti (Immunology Unit, “G. Gaslini” Institute, Genoa, Italy). The pNifty reporter plasmid (InvivoGen) expresses the secreted embryonic alkaline phosphatase (SeAP) gene under the control of the NF-κB–inducible endothelial leukocyte adhesion molecule-1 composite promoter, which enables quantification of NF-κB activation by measuring SeAP activity in media containing the specific enzyme substrate. HEK293T4 and control untransfected HEK293 cells were grown in DMEM (Invitrogen) containing 10% FBS (Gibco-BRL).
For in vitro experiments, human purified monocytes, HUVECs, HEK293, HEK293T4, and THP1 cells were treated with human purified anti-β2GPI peptide, anti-BSA Abs, or Escherichia coli (E coli) lipopolysaccharide (LPS; Sigma-Aldrich).
Functional experiments were also carried out by preincubating cells with polymyxin B (10 μg/mL; Sigma-Aldrich) to exclude the possibility of LPS contamination. In parallel experiments, human monocytes and HUVECs were also incubated with purified IgG from an APS patient, which was positive for β2GPI peptide (referred to as APS IgG). IgG was obtained from the serum of an APS patient by precipitation with 33% ammonium sulfate.34
Anti-β2GPI peptide Ab binding to β2GPI and TLR4
Native β2GPI purified from human plasma (5 μg; Calbiochem) was run in 10% SDS-PAGE in reducing conditions and electrophoretically transferred to a nitrocellulose membrane (Bio-Rad). Blots were probed with human purified anti-β2GPI peptide and anti-BSA Abs, followed by HRP-conjugated goat anti–human IgG (Bio-Rad). 3,3′-diaminobenzidine dihydrochloride (Sigma-Aldrich) was used as a substrate. The HEK293T4 and control untransfected HEK293 cells were used to detect TLR4 with Western blotting and indirect immunofluorescence. Cell lysates were run in 7.5% SDS-PAGE under reducing conditions and blotted. Blots were probed with rabbit anti–human TLR4 polyclonal Abs (Antibodies-online), human purified anti-β2GPI peptide, and anti-BSA Abs, followed, respectively, by HRP-conjugated goat anti–rabbit IgG and anti–human IgG Abs (Bio-Rad). The immunoreactivity was assessed by chemiluminescence reaction using the enhanced chemiluminescence (ECL) Western blotting system (Amersham Pharmacia Biotech). For indirect immunofluorescence, the cells were fixed in PBS containing 4% formaldehyde for 30 minutes at 4°C. After washing with PBS, cells were incubated for 1 hour at 4°C with human purified anti-β2GPI peptide Abs or with control human purified anti-BSA Abs (0.1 μg/μL) in PBS containing 1% BSA. Texas Red–conjugated anti–human IgG (Pierce) were then added and incubated at 4°C for 30 minutes. Alternatively, cells were incubated with monoclonal anti-TLR4–FITC Abs (Abcam) for 1 hour at 4°C. After washing with PBS, images were acquired with a scanning confocal microscope (Leica Microsystems) equipped with an argon ion laser. FITC and Texas Red fluorochromes were excited at 488 nm and 518 nm, respectively. Images were collected at 512 × 512 pixels, processed, and filtered to minimize background.
Immunoprecipitation
Cell-free lysates from HEK293T4 cells were immunoprecipitated with human purified anti-β2GPI peptide Abs. In brief, cells were lysed in lysis buffer containing 20mM HEPES, pH 7.2, 1% Nonidet P-40, 10% glycerol, 50mM NaF, including protease inhibitors (Sigma-Aldrich). To preclear nonspecific binding, cell-free lysates were mixed with protein A–Sepharose beads (Bio-Rad) and stirred in a rotary shaker for 1 hour at 4°C. After centrifugation (500g for 1 minute), the supernatant was immunoprecipitated with human purified anti-β2GPI peptide Abs (3 μg) or with irrelevant IgG as a negative control (3 μg) plus protein A–Sepharose beads. The immunoprecipitates were subjected to 7.5% SDS-PAGE and immunoblotting with anti-TLR4 polyclonal Abs (Antibodies-online). Immunoreactivity was assessed by the chemiluminescence reaction using the ECL Western blotting system (Amersham).
Activation of TLR4 by Abs
HEK293T4 cells (2.5 × 104 cells/well) were grown to 60%-70% confluence in 96-well microplates (Costar; Corning) and treated for 24 hours with E coli LPS (1 ng/mL, 100 and 10 pg/mL; Sigma-Aldrich) as a positive control for TLR4 activation; human purified anti-β2GPI peptide Abs (100, 50, and 10 μg/mL); and human purified anti-BSA Abs (100, 50, and 10 μg/mL) as a negative control. After treatment, cells were resuspended in QUANTI-Blue Detection Medium (InvivoGen) to monitor the NF-κB activation induced by TLR4 signaling.35 This medium, which was specifically designed for the detection of NF-κB activation, contains a specific SeAP substrate and turns blue in the presence of phosphatase activity. The blue color was assessed by a spectrophotometer set at 620 nm. Results are expressed as a percentage of the positive control, where the positive control is the OD value obtained on stimulation of HEK293T4 cells with E coli LPS at 100 pg/mL (ie, the maximal concentration used). Experiments to inhibit TLR4 activation were carried out by incubating human purified anti-β2GPI peptide Abs (100 μg/mL) with different concentrations of the peptide PDDLPFST and with the unrelated peptide DSPLTFDP (80, 60, 40, 20, 10, and 5 μg/mL) for 1 hour at 37°C.
TLR4 knockdown by siRNA
THP1 cells were seeded (3 × 105 cells/dish) in 60-mm dishes. Twenty-four hours after seeding, cells were transfected with GeneSolution siRNA (QIAGEN) according to the manufacturer's instructions using 120 pmol/μL of Hs_TLR4_10 (AACCCGGAGGCCATTATGCTA) and Hs_TLR4_11 (AAGAGGGTACCTCTCATGTTA; both QIAGEN). As an experimental control, cells were also transfected with 120 pmol/μL of nonsilencing siRNA (AllStars Negative Control; QIAGEN). The effect of transfection after 48 hours was verified by flow cytometric analysis using FITC-conjugated mouse anti–human TLR4 mAb (Abcam).
IRAK phosphorylation assay
For the interleukin receptor-associated kinase (IRAK) phosphorylation assay, monocytes, HUVECs and THP1 cells (both TLR4 silenced and not silenced) were stimulated for 45 minutes with human purified anti-β2GPI peptide (100 μg/mL), human purified anti-BSA Abs (100 μg/mL), and E coli LPS (100 ng/mL).
Monocytes and HUVECs were also treated with APS IgG (200 μg/mL) or the same amount of APS IgG preabsorbed with β2GPI peptide. After treatment, cells were placed on ice, washed once in PBS, and scraped.
For the preparation of whole-cell extracts, cells were resuspended in lysis buffer containing 20mM HEPES, pH 7.2, 1% Nonidet P-40, 10% glycerol, 50mM NaF, 1mM Na3VO4, including protease inhibitors (Sigma-Aldrich). The protein content was determined by Bradford assay (Sigma-Aldrich). Equal amounts of whole-extract proteins were separated in 7.5% SDS-PAGE and blotted onto nitrocellulose membranes (Bio-Rad). Polyclonal anti–phospho-IRAK1 Abs (Cell Signaling Technology) and HRP-conjugated goat anti–rabbit IgG Abs (Bio-Rad) were used. Immunoreactivity was assessed by the chemiluminescence reaction using the ECL Western blotting system (Amersham Pharmacia Biotech). As a loading control, IRAK1-blotted membranes were stripped and reprobed with polyclonal anti–α-actin Abs (Sigma-Aldrich).
NF-κB assay
The NF-κB p65/p50 transcription factor assay kit (Alexis Biochemicals) was used to monitor the activation of NF-κB. After incubation for 45 minutes with human anti-β2GPI peptide, anti-BSA Abs (100 μg/mL), E coli LPS (100 ng/mL; Sigma-Aldrich), APS IgG (200 μg/mL), or APS IgG (200 μg/mL) preabsorbed with β2GPI peptide, the monocytes, HUVECs, and THP1 cells (both TLR4 silenced and not silenced) were resuspended in lysis buffer. Alternatively, lysates were obtained from cells that had been pretreated for 30 minutes with the synthetic MEK inhibitor PD98059 (10μM; EMD Biosciences) and then stimulated as previously described. The protein contents were quantified with the Bradford assay (Sigma-Aldrich) and equal amounts of lysates were used to test the levels of activated p50 and p65 subunits in the presence of the Abs directed against the subunits bound to the oligonucleotide containing the NF-κB consensus-binding site. HeLa cell extract was used as a positive control, either alone or in the presence of wild-type or mutated consensus oligonucleotide.
VCAM expression on endothelial cells
After treatment with E coli LPS (Sigma-Aldrich), human purified endotoxin-free anti-β2GPI peptide, or anti-BSA Abs, VCAM expression was analyzed by flow cytometry on HUVEC cell surfaces. Briefly, endothelial cells were seeded in 12-well microplates (Costar; Corning) and allowed to achieve confluence. Cells were then treated for 6 hours with E coli LPS (100 ng/mL; Sigma-Aldrich), anti-β2GPI peptide, or anti-BSA Abs (100 μg/mL). VCAM expression was detected using anti-VCAM mAb (Chemicon International) and FITC-conjugated goat anti–mouse IgG as a secondary Ab (Sigma-Aldrich).
TNF-α production by monocytes
Supernatants were collected from monocytes incubated for 48 hours with human purified endotoxin-free anti-β2GPI peptide or anti-BSA Abs (100 μg/mL) or E coli LPS (100 ng/mL; Sigma-Aldrich). TNF-α levels in the medium were determined by ELISA using a commercially available ELISA kit (OptEIA Sets; BD Pharmingen) according to the manufacturer's instructions.
The incubation time of 48 hours was selected according to our previous study.22 In the present study, we preliminarily analyzed the release of TNF-α after 12, 24, and 48 hours, and the results were virtually the same.
Preliminary experiments were also designed to determine the detection limits and the linearity and range of the ELISA, essentially in accordance with the International Conference on Harmonization Q2A and Q2B guidelines (Committee for Proprietary Medicinal Products, European Medicines Evaluation Agency). The intra-assay variation ranged from 3%-6% and the inter-assay variation ranged from 4%-9%. The limit of detection were 16 pg/mL.
Statistical analysis
The χ2 test was used to evaluate differences between percentages, and the Mann-Whitney unpaired test or the Student paired t test were used to compare quantitative variables. Linear regression analysis (r correlation coefficient) was used to identify significant correlations. P < .05 was considered significant. All cell cultures were performed in triplicate. Flow cytometry data were evaluated statistically using the Kolmogorov-Smirnov test,36 and a D/s(n) ratio ≥ 15 was accepted as significant in the experimental conditions used.
Results
Identification of a peptide within β2GPI domain I with amino acid sequence homology to TLR4
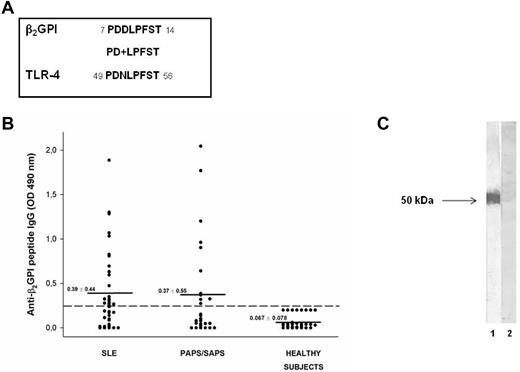
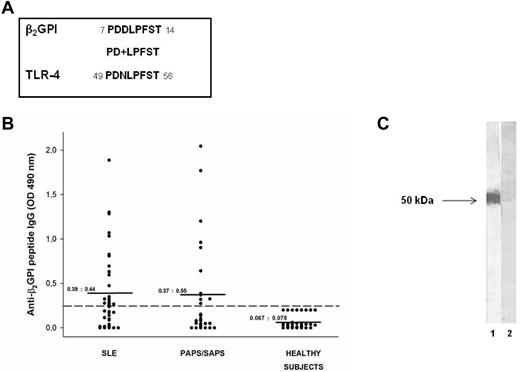
To investigate the possible antigenic cross-reactivity between anti-β2GPI domain I Abs and self-proteins potentially involved in inflammatory or coagulant events, we analyzed the amino acid sequence of β2GPI domain I divided into segments of approximately 15 residues with 5 residues overlapping in a human protein databank (the Swiss-Prot database of human sequences), using BLASTP via the NCBI BLAST network service. We identified a peptide (PDDLPFST) sharing 88% identity with an epitope of the extracellular region of human TLR4 (PDNLPFST) and an E value = 0.005 (Figure 1A).
Molecular and immunologic characterization of the β2GPI peptide. (A) Sequence homology between β2GPI peptide and TLR4 (+ indicates conservative substitutions). (B) Immunoreactivity against β2GPI peptide in patients with SLE, primary APS (PAPS), and APS secondary to SLE (SAPS) and in sex- and age-matched healthy subjects. The broken line represents the cutoff (mean + 2 SDs for healthy subjects) and the mean OD values for each group analyzed are indicated. Results are expressed as absorbance at 490 nm. (C) The reactivity of human purified anti-β2GPI peptide and anti-BSA Abs with β2GPI was analyzed by Western blot. Human blood–purified β2GPI was probed with human purified anti-β2GPI peptide (lane 1) and anti-BSA IgG (lane 2), followed by HRP-linked goat anti–human IgG Abs. 3,3′-diaminobenzidine dihydrochloride was used as a substrate.
Molecular and immunologic characterization of the β2GPI peptide. (A) Sequence homology between β2GPI peptide and TLR4 (+ indicates conservative substitutions). (B) Immunoreactivity against β2GPI peptide in patients with SLE, primary APS (PAPS), and APS secondary to SLE (SAPS) and in sex- and age-matched healthy subjects. The broken line represents the cutoff (mean + 2 SDs for healthy subjects) and the mean OD values for each group analyzed are indicated. Results are expressed as absorbance at 490 nm. (C) The reactivity of human purified anti-β2GPI peptide and anti-BSA Abs with β2GPI was analyzed by Western blot. Human blood–purified β2GPI was probed with human purified anti-β2GPI peptide (lane 1) and anti-BSA IgG (lane 2), followed by HRP-linked goat anti–human IgG Abs. 3,3′-diaminobenzidine dihydrochloride was used as a substrate.
Serum IgG reactivity against the identified peptide of β2GPI
We analyzed by ELISA the serum IgG reactivity against the identified peptide of β2GPI (PDDLPFST) in patients with APS and SLE and in healthy subjects. ELISA detected serum IgG specific for the identified peptide in 11 of 27 (41%) patients with APS (primary APS and APS secondary to SLE) and in 22 of 44 (50%) patients with SLE, but not in healthy subjects (Figure 1B). The preabsorption with peptide of the sera from 2 patients positive for anti-β2GPI peptide Abs completely inhibited the Ab reactivity, confirming the specificity of the ELISA (data not shown).
We found no significant association between serum anti-β2GPI peptide Abs and age, sex, disease duration, or other clinical characteristics considered for APS and SLE (Tables 1 and 2). No association between the presence of serum anti-β2GPI peptide IgG and anti-β2GPI IgG or anti-cardiolipin/β2GPI IgG was found. In particular, we observed that 23 of 33 (70%) patients positive for anti-β2GPI peptide IgG were negative for anti-β2GPI and anti-cardiolipin/β2GPI IgG.
We also observed that anti-β2GPI peptide Abs were not able to bind β2GPI, either alone or in combination with cardiolipin, in the ELISA assay (data not shown), but were able to bind blood-purified β2GPI in Western blotting (Figure 1C). These observations suggest that the peptide is cryptic within the β2GPI structure and remains hidden, even after β2GPI binding to phospholipids, and that anti-β2GPI peptide Abs might arise by a mechanism of molecular mimicry.
Identification of a P gingivalis epitope sharing complete identity with the β2GPI peptide
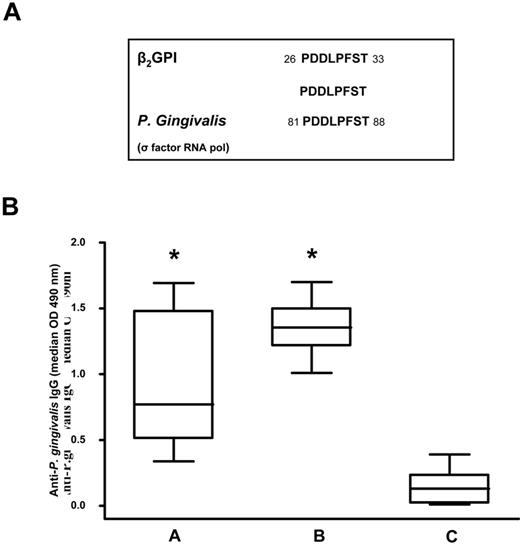
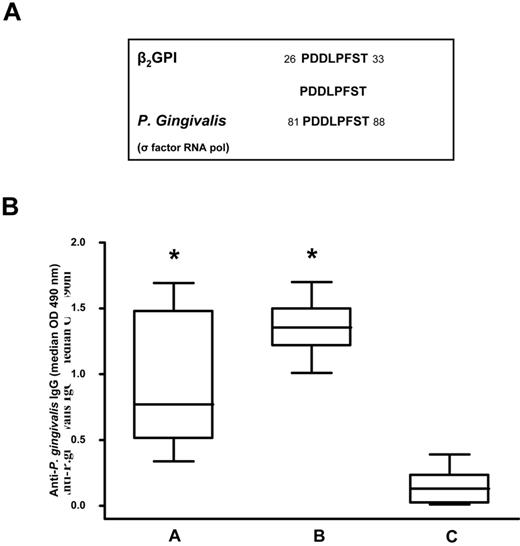
Because previous studies demonstrated that β2GPI shares epitope homology with certain microbial pathogens37 such as periodontal pathogens,38 we compared the peptide sequence with known microbial sequences in the Swiss-Prot database using BLASTP. We found that the peptide sequence shared a complete identity (100%) with an epitope of P gingivalis RNA polymerase σ-70 factor (PDDLPFST; Figure 2A).
Sequence homology between the β2GPI peptide and P gingivalis and anti–P gingivalis immunoreactivity. (A) Sequence homology between β2GPI peptide and P gingivalis. (B) Box-whisker plots of anti–P gingivalis IgG in patients with SLE and APS positive for anti-β2GPI peptide IgG (A group), subjects with moderate chronic periodontitis (B group), and healthy subjects without periodontitis and autoimmune diseases (C group). Median, quartiles, range, and possibly extreme values are indicated. Statistical analysis was performed with the Mann-Whitney test. *P < .001 compared with the C group.
Sequence homology between the β2GPI peptide and P gingivalis and anti–P gingivalis immunoreactivity. (A) Sequence homology between β2GPI peptide and P gingivalis. (B) Box-whisker plots of anti–P gingivalis IgG in patients with SLE and APS positive for anti-β2GPI peptide IgG (A group), subjects with moderate chronic periodontitis (B group), and healthy subjects without periodontitis and autoimmune diseases (C group). Median, quartiles, range, and possibly extreme values are indicated. Statistical analysis was performed with the Mann-Whitney test. *P < .001 compared with the C group.
Using ELISA, we analyzed serum IgG levels specific for P gingivalis in 23 patients positive for anti-β2GPI peptide IgG (group A), in 16 subjects with moderate chronic periodontitis (group B), and in 20 healthy subjects without periodontitis and autoimmune diseases (group C). We observed that patients in the A and B groups had a significantly higher prevalence of IgG (P < .001) against P gingivalis (Figure 2B) than the C group. Serum samples from all subjects with moderate chronic periodontitis and healthy subjects without periodontitis and autoimmune diseases were negative for anti-β2GPI peptide, anti-β2GPI, and anti-cardiolipin/β2GPI IgG.
Anti-β2GPI peptide Abs bind TLR4
To determine whether anti-β2GPI peptide IgGs were able to bind TLR4, we analyzed HEK293T4 and control untransfected HEK293 cells by Western blot and fluorescence microscopy.
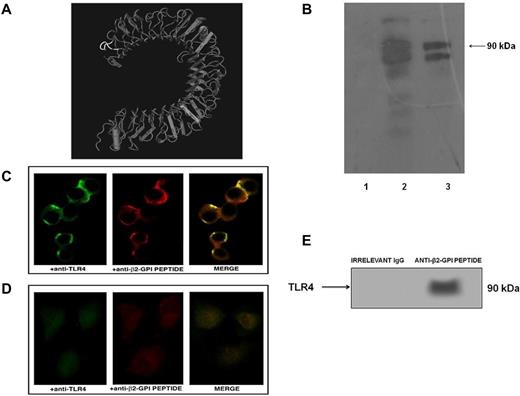
Western blot analysis revealed that human purified anti-β2GPI peptide Abs cross-reacted with human TLR4, as assessed by 2 closely related, approximately 90-kDa bands that could reflect different degrees of TLR4 glycosylation (Figure 3B). Immunofluorescence analysis with human anti-β2GPI peptide Abs and with anti-TLR4 mAbs disclosed similar distribution patterns. The merged images showed nearly complete colocalization areas on the surfaces of transfected cells (Figure 3C). Human purified anti-β2GPI peptide Abs were not able to stain untransfected cells (Figure 3D), and human purified anti-BSA Abs were not able to stain either HEK293T4 or control untransfected HEK293 cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Anti-β2GPI peptide Abs bind human TLR4. (A) Crystal structure of TLR4. The sequence of the PDDLPFST peptide is highlighted (white). (B) Immunoreactivity of anti-β2GPI peptide Abs to TLR4 was analyzed by Western blot. Cell lysates from HEK293T4 cells were probed with human purified anti-BSA IgG (lane 1), rabbit anti–human TLR4 Abs (lane 2), and human purified anti-β2GPI peptide IgG (lane 3), followed by HRP-linked goat anti–human or anti–rabbit IgG Abs. Immunoreactivity was assessed by ECL. (C) Fluorescent confocal microscopy images showing a coimmunolocalization of TLR4 (green) and β2GPI peptide (red) on the HEK293T4 cell surface using monoclonal anti-TLR4–FITC conjugated and human purified anti-β2GPI peptide Abs (0.1 μg/μL), followed by Texas Red–conjugated anti–human IgG Abs. Merged image (overlay) depicts areas of overlap in yellow. (D) Fluorescent confocal microscopy images of untransfected HEK293 cells as a negative control. (E) HEK293T4 cells were immunoprecipitated with human anti-β2GPI peptide Abs. The immunoprecipitates were analyzed by Western blotting using polyclonal anti-TLR4 Abs. Bound Abs were visualized with HRP-conjugated anti–rabbit IgG and immunoreactivity was assessed by ECL. Virtually, no reactivity was found with immunoprecipitates obtained using control irrelevant IgG.
Anti-β2GPI peptide Abs bind human TLR4. (A) Crystal structure of TLR4. The sequence of the PDDLPFST peptide is highlighted (white). (B) Immunoreactivity of anti-β2GPI peptide Abs to TLR4 was analyzed by Western blot. Cell lysates from HEK293T4 cells were probed with human purified anti-BSA IgG (lane 1), rabbit anti–human TLR4 Abs (lane 2), and human purified anti-β2GPI peptide IgG (lane 3), followed by HRP-linked goat anti–human or anti–rabbit IgG Abs. Immunoreactivity was assessed by ECL. (C) Fluorescent confocal microscopy images showing a coimmunolocalization of TLR4 (green) and β2GPI peptide (red) on the HEK293T4 cell surface using monoclonal anti-TLR4–FITC conjugated and human purified anti-β2GPI peptide Abs (0.1 μg/μL), followed by Texas Red–conjugated anti–human IgG Abs. Merged image (overlay) depicts areas of overlap in yellow. (D) Fluorescent confocal microscopy images of untransfected HEK293 cells as a negative control. (E) HEK293T4 cells were immunoprecipitated with human anti-β2GPI peptide Abs. The immunoprecipitates were analyzed by Western blotting using polyclonal anti-TLR4 Abs. Bound Abs were visualized with HRP-conjugated anti–rabbit IgG and immunoreactivity was assessed by ECL. Virtually, no reactivity was found with immunoprecipitates obtained using control irrelevant IgG.
To further confirm the Ab cross-reactivity between β2GPI peptide and TLR4, we preabsorbed anti-β2GPI peptide Abs with human recombinant TLR4 at different concentrations (5-20 μg/mL). We observed a dose-dependent inhibition in ELISA (data not shown), reaching 100% with the maximal concentration of TLR4 (OD = 0.85 for unabsorbed anti-β2GPI peptide Abs vs OD = 0.05 for TLR4 preabsorbed anti-β2GPI peptide Abs).
To further support the anti-β2GPI peptide Abs binding to TLR4, we demonstrated that anti-β2GPI peptide Abs immunoprecipitated TLR4 in HEK293T4 cells, as revealed by Western blot analysis (Figure 3E).
Anti-β2GPI peptide Abs activate TLR4 in cells stably transfected with TLR4 gene
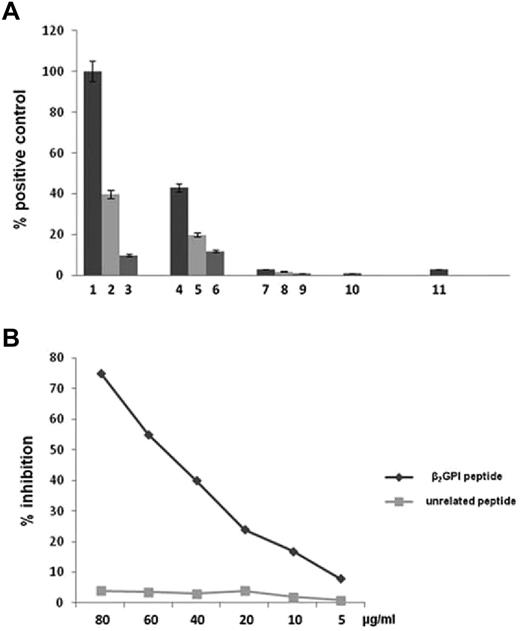
To study the ability of anti-β2GPI peptide Abs to engage TLR4 and activate NF-κB, HEK293T4 cells stably transfected with genes encoding the coreceptors MD2 and CD14 and cotransfected with the pNifty plasmid were stimulated in the presence of E coli LPS or endotoxin-free human purified anti-β2GPI peptide and anti-BSA Abs. Anti-β2GPI peptide Abs were able to activate TLR4, but anti-BSA Abs and serum deprived of anti-β2GPI peptide Abs had no effect (Figure 4A). The activation of TLR4 by anti-β2GPI peptide Abs was inhibited in a dose-dependent manner by β2GPI peptide PDDLPFST, but not by the unrelated control peptide DSPLTFDP (Figure 4B).
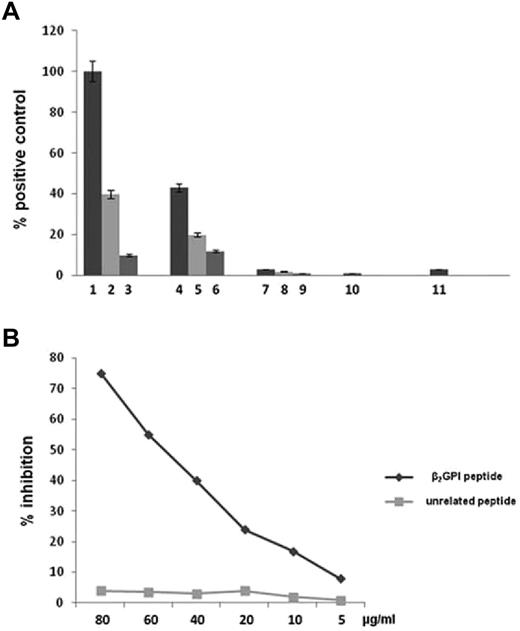
Anti-β2GPI peptide Abs activate TLR4. (A) HEK293T4 cells were treated for 24 hours with E coli LPS 1 ng/mL (bar 1), 100 pg/mL (bar 2), and 10 pg/mL (bar 3); endotoxin-free human anti-β2GPI peptide Abs 100 μg/mL (bar 4), 50 μg/mL (bar 5), and 10 μg/mL (bar 6); endotoxin-free human anti-BSA Abs 100 μg/mL (bar 7), 50 μg/mL (bar 8), and 10 μg/mL (bar 9); serum deprived of anti-β2GPI peptide Abs 100 μg/mL (bar 10); and untreated cells (bar 11). Results are expressed as a percentage of positive control, where the positive control is the mean OD value obtained on stimulation of HEK293T4 cells with 1 ng/mL of E coli LPS. Values are the means ± SDs of 3 different experiments. (B) Inhibition of HEK293T4 cell activation (NF-κB) induced by human anti-β2GPI peptide Abs using β2GPI peptide or an unrelated peptide. The y-axis represents the percentage of inhibition, and the x-axis indicates inhibitor concentrations.
Anti-β2GPI peptide Abs activate TLR4. (A) HEK293T4 cells were treated for 24 hours with E coli LPS 1 ng/mL (bar 1), 100 pg/mL (bar 2), and 10 pg/mL (bar 3); endotoxin-free human anti-β2GPI peptide Abs 100 μg/mL (bar 4), 50 μg/mL (bar 5), and 10 μg/mL (bar 6); endotoxin-free human anti-BSA Abs 100 μg/mL (bar 7), 50 μg/mL (bar 8), and 10 μg/mL (bar 9); serum deprived of anti-β2GPI peptide Abs 100 μg/mL (bar 10); and untreated cells (bar 11). Results are expressed as a percentage of positive control, where the positive control is the mean OD value obtained on stimulation of HEK293T4 cells with 1 ng/mL of E coli LPS. Values are the means ± SDs of 3 different experiments. (B) Inhibition of HEK293T4 cell activation (NF-κB) induced by human anti-β2GPI peptide Abs using β2GPI peptide or an unrelated peptide. The y-axis represents the percentage of inhibition, and the x-axis indicates inhibitor concentrations.
Anti-β2GPI peptide Abs induced monocyte activation
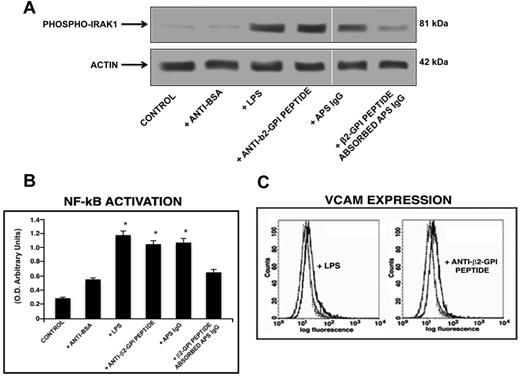
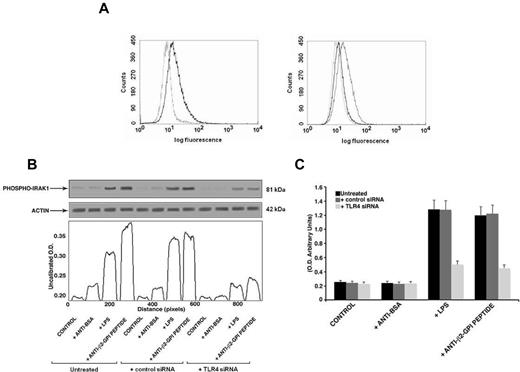
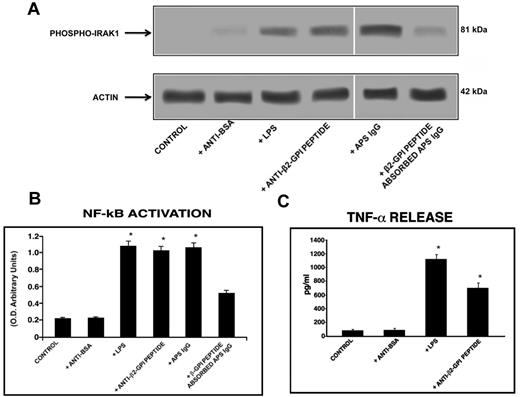
Western blot analysis of cell lysates showed that human purified endotoxin-free anti-β2GPI peptide Abs, as well as E coli LPS and the IgG fraction from the serum of an APS patient positive for the β2GPI peptide, induced IRAK phosphorylation, as revealed by anti–phospho-IRAK1 Abs reactivity. Conversely, cells stimulated with human purified anti-BSA Abs did not show any effect. Interestingly, anti–phospho-IRAK reactivity was inhibited by preabsorption of APS IgG with β2GPI peptide (Figure 5A).
Anti-β2GPI peptide Abs activate monocytes. (A) IRAK phosphorylation was evaluated by Western blot. Monocytes were treated for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); with E coli LPS (100 ng/mL); with purified IgG from an APS patient positive for the β2GPI peptide (200 μg/mL); or with APS IgG (200 μg/mL) preabsorbed with β2GPI peptide. Whole-cell lysates were probed with anti–phospho-IRAK1 and anti–α-actin polyclonal Abs as a loading control. Bound Abs were visualized with HRP-linked goat anti–rabbit IgG and immunoreactivity was assayed by ECL. (B) NF-κB activation was analyzed by treating monocytes for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); with E coli LPS (100 ng/mL); with APS IgG (200 μg/mL); or with APS IgG (200 μg/mL) preabsorbed with β2GPI peptide. Equal amounts of whole-cell lysates were used to determine the levels of the activated p65 subunit using Abs directed against the subunit bound to the oligonucleotide containing the NF-κB consensus-binding site. Values are the means ± SDs of 3 different experiments. Statistical analysis was performed by the Student paired t test (P < .001). * indicate versus control. (C) Release of TNF-α was analyzed using a commercially available ELISA kit. Cells were stimulated for 48 hours with human purified endotoxin-free anti-BSA and anti-β2GPI peptide Abs (100 μg/mL) and E coli LPS (100 ng/mL) and, after the treatment, supernatants were collected and analyzed. Values are the means ± SDs of 3 different experiments. Statistical analysis was performed by the Student paired t test (P < .001). * indicates versus control.
Anti-β2GPI peptide Abs activate monocytes. (A) IRAK phosphorylation was evaluated by Western blot. Monocytes were treated for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); with E coli LPS (100 ng/mL); with purified IgG from an APS patient positive for the β2GPI peptide (200 μg/mL); or with APS IgG (200 μg/mL) preabsorbed with β2GPI peptide. Whole-cell lysates were probed with anti–phospho-IRAK1 and anti–α-actin polyclonal Abs as a loading control. Bound Abs were visualized with HRP-linked goat anti–rabbit IgG and immunoreactivity was assayed by ECL. (B) NF-κB activation was analyzed by treating monocytes for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); with E coli LPS (100 ng/mL); with APS IgG (200 μg/mL); or with APS IgG (200 μg/mL) preabsorbed with β2GPI peptide. Equal amounts of whole-cell lysates were used to determine the levels of the activated p65 subunit using Abs directed against the subunit bound to the oligonucleotide containing the NF-κB consensus-binding site. Values are the means ± SDs of 3 different experiments. Statistical analysis was performed by the Student paired t test (P < .001). * indicate versus control. (C) Release of TNF-α was analyzed using a commercially available ELISA kit. Cells were stimulated for 48 hours with human purified endotoxin-free anti-BSA and anti-β2GPI peptide Abs (100 μg/mL) and E coli LPS (100 ng/mL) and, after the treatment, supernatants were collected and analyzed. Values are the means ± SDs of 3 different experiments. Statistical analysis was performed by the Student paired t test (P < .001). * indicates versus control.
Because IRAK phosphorylation leads to NF-κB activation, we investigated the effects of human anti-β2GPI peptide Abs on p50 and p65 NF-κB in monocytes and endothelial cells. Active p65 levels in monocytes stimulated with anti-β2GPI peptide Abs (P < .001) and APS IgG (P < .001) increased significantly, compared with untreated monocytes or monocytes stimulated with human anti-BSA Abs. These activation levels were only slightly lower than those obtained with E coli LPS stimulation (Figure 5B). Active p65 levels were reduced significantly (P = .001) by preabsorption of APS IgG with β2GPI peptide (Figure 5B). Similar findings were found for active p50 levels (data not shown).
The specificity of the assay was assessed by the incubation of HeLa cell extract in the presence of a unbound wild-type consensus oligonucleotide that abolished the binding of both subunits, whereas the incubation of the HeLa cell extract with mutated consensus oligonucleotide left NF-κB binding unchanged (data not shown).
Anti-β2GPI peptide Abs induce TNF-α release
Monocytes stimulated with human purified anti-β2GPI peptide Abs released a significantly (P < .05) higher level of the proinflammatory cytokine TNF-α in the cell-culture supernatant compared with untreated monocytes and/or monocytes stimulated with human purified endotoxin-free anti-BSA Abs. The amount of TNF-α released was only slightly lower than that obtained with E coli LPS stimulation (Figure 5C).
Anti-β2GPI peptide Abs induce endothelial cell activation
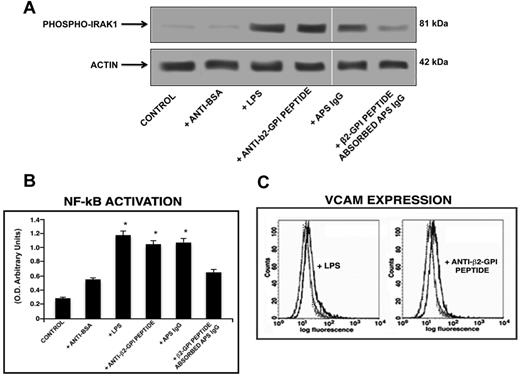
The treatment of HUVECs with anti-β2GPI peptide Abs and with the E coli LPS and IgG fractions from the serum of an APS patient resulted in IRAK phosphorylation, as shown by Western blot analysis (Figure 6A). In addition, HUVECs stimulated with anti-β2GPI peptide Abs (P = .001), E coli LPS and IgG fractions from APS (P < .001) showed a significant increase of active p65 NF-κB, compared with untreated HUVECs or HUVECs stimulated with human purified endotoxin-free anti-BSA Abs (Figure 6B).
Anti-β2GPI peptide Abs activate endothelial cells. (A) IRAK phosphorylation was evaluated by Western blot analysis. Endothelial cells were incubated for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); E coli LPS (100 ng/mL); with purified IgG from an APS patient positive for the β2GPI peptide (200 μg/mL); or with APS IgG (200 μg/mL) preabsorbed with β2GPI peptide. (B) NF-κB activation was analyzed by treating endothelial cells for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); E coli LPS (100 ng/mL); APS IgG (200 μg/mL); or with APS IgG (200 μg/mL) preabsorbed with β2GPI peptide. Values are the means ± SDs of 3 different experiments. Statistical analysis was performed by Student paired t test (P < .001). * indicates versus control. (C) VCAM expression on the cell surface was evaluated by flow cytometric analysis. Representative flow cytometry histogram plots show the fluorescence intensity of FITC-conjugated anti-VCAM mAbs after treatment of HUVECs with E coli LPS or endotoxin-free human purified anti-β2GPI peptide Abs. Isotype control staining is represented by the dotted line; anti-VCAM-labeled cells are represented by the gray (untreated cells) and the black (treated cells) lines. Statistical differences between the peaks of cells were evaluated by the Kolmogorov-Smirnov test. D/s(n) ratio > 15 for treated versus untreated cells.
Anti-β2GPI peptide Abs activate endothelial cells. (A) IRAK phosphorylation was evaluated by Western blot analysis. Endothelial cells were incubated for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); E coli LPS (100 ng/mL); with purified IgG from an APS patient positive for the β2GPI peptide (200 μg/mL); or with APS IgG (200 μg/mL) preabsorbed with β2GPI peptide. (B) NF-κB activation was analyzed by treating endothelial cells for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); E coli LPS (100 ng/mL); APS IgG (200 μg/mL); or with APS IgG (200 μg/mL) preabsorbed with β2GPI peptide. Values are the means ± SDs of 3 different experiments. Statistical analysis was performed by Student paired t test (P < .001). * indicates versus control. (C) VCAM expression on the cell surface was evaluated by flow cytometric analysis. Representative flow cytometry histogram plots show the fluorescence intensity of FITC-conjugated anti-VCAM mAbs after treatment of HUVECs with E coli LPS or endotoxin-free human purified anti-β2GPI peptide Abs. Isotype control staining is represented by the dotted line; anti-VCAM-labeled cells are represented by the gray (untreated cells) and the black (treated cells) lines. Statistical differences between the peaks of cells were evaluated by the Kolmogorov-Smirnov test. D/s(n) ratio > 15 for treated versus untreated cells.
Anti-β2GPI peptide Abs induce VCAM expression on endothelial cells
We also investigated the response of HUVECs to treatment with human anti-β2GPI peptide Abs in terms of expression of VCAM on the cell surface. Cells were stimulated with E coli LPS, human anti-β2GPI peptide, and anti-BSA Abs, and VCAM expression was evaluated by flow cytometry. VCAM expression was detected on the cell surface of HUVECs stimulated with E coli LPS and HUVECs stimulated with anti-β2GPI peptide Abs, but not on unstimulated cells or cells stimulated with anti-BSA Abs (Figure 6C). Values of the mean fluorescence intensity ratio (obtained with the value given by specific Abs above the value given by control Abs) were 1.6 ± 0.2 and 1.4 ± 0.1 for LPS and anti-β2GPI peptide Abs treatment, respectively. Parallel experiments were carried out with anti-BSA Abs and, in all conditions tested, no changes of VCAM expression were found compared with untreated cells (data not shown).
Effect of TLR4 siRNA on TLR4 expression, IRAK phosphorylation, and NF-κB activation
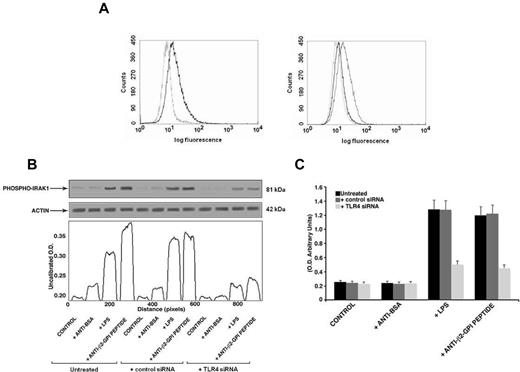
Finally, to further confirm the specific role of TLR4 in anti-β2GPI peptide Ab-mediated activation, we transfected THP1 cells with TLR4 siRNA. We found a significant reduction of TLR4 surface expression in transfected cells with respect to nonsilencing siRNA transfected cells (mean fluorescence intensity ratio for TLR4 expression: 2 ± 0.1 vs 1.23 ± 0.3, P = .014, Figure 7A). In anti-β2GPI peptide Ab-treated cells, knocking down TLR4 reduced both IRAK phosphorylation, as revealed by densitometric analysis (Figure 7B), and NF-κB activation (Figure 7C).
Effects of TLR4 knockdown. (A) Flow cytometric analysis of cell surface TLR4 expression 48 hours after siRNA transfection (left panel: not transfected cells; right panel: AllStars negative control siRNA- and TLR4 siRNA-transfected cells). The dotted line represents isotype control; the black line represents TLR4 expression in nontransfected and TLR4 siRNA-transfected cells; the gray line represents TLR4 expression in negative control transfected cells (AllStars siRNA). A representative experiment of 3 is shown. (B) IRAK phosphorylation was evaluated by Western blot analysis. THP1 cells (silenced with TLR4 siRNA and control siRNA) were treated for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); or with E coli LPS (100 ng/mL). Cell lysates were probed with anti–phospho-IRAK1 and anti–α-actin polyclonal Abs as a loading control. Bound Abs were visualized with HRP-linked goat anti–rabbit IgG and immunoreactivity was assayed by ECL. Scanning densitometric analysis of each sample is shown. (C) NF-κB activation was analyzed by treating THP1 cells (silenced with TLR4 siRNA and control siRNA) for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL) or with E coli LPS (100 ng/mL). Equal amounts of whole-cell lysates were used to determine the levels of the activated p65 subunit using Abs directed against the subunit bound to the oligonucleotide containing the NF-κB consensus-binding site. Values are the means ± SDs of 3 different experiments.
Effects of TLR4 knockdown. (A) Flow cytometric analysis of cell surface TLR4 expression 48 hours after siRNA transfection (left panel: not transfected cells; right panel: AllStars negative control siRNA- and TLR4 siRNA-transfected cells). The dotted line represents isotype control; the black line represents TLR4 expression in nontransfected and TLR4 siRNA-transfected cells; the gray line represents TLR4 expression in negative control transfected cells (AllStars siRNA). A representative experiment of 3 is shown. (B) IRAK phosphorylation was evaluated by Western blot analysis. THP1 cells (silenced with TLR4 siRNA and control siRNA) were treated for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL); or with E coli LPS (100 ng/mL). Cell lysates were probed with anti–phospho-IRAK1 and anti–α-actin polyclonal Abs as a loading control. Bound Abs were visualized with HRP-linked goat anti–rabbit IgG and immunoreactivity was assayed by ECL. Scanning densitometric analysis of each sample is shown. (C) NF-κB activation was analyzed by treating THP1 cells (silenced with TLR4 siRNA and control siRNA) for 45 minutes with human purified endotoxin-free anti-β2GPI peptide and, as controls, with anti-BSA Abs (100 μg/mL) or with E coli LPS (100 ng/mL). Equal amounts of whole-cell lysates were used to determine the levels of the activated p65 subunit using Abs directed against the subunit bound to the oligonucleotide containing the NF-κB consensus-binding site. Values are the means ± SDs of 3 different experiments.
Discussion
In this study, we demonstrated for the first time the presence of autoantibodies specific to a peptide of β2GPI domain I sharing a high degree of homology with an extracellular epitope of human TLR4 in a high percentage of sera from patients with SLE and APS, but not in sera from healthy donors. Our results provide evidence that these Abs, triggering a TLR4 signaling cascade with consequent IRAK phosphorylation and NF-κB translocation, promote VCAM expression on endothelial cells and TNF-α release by monocytes, thus leading to a proinflammatory microenvironment.
Previously, Vlachoyiannopoulos et al found that the same β2GPI peptide studied herein (aa 7-13) shares homology with a peptide of the CD40 intracellular region.39 The investigators suggested that Abs binding this peptide could react and potentially activate different cells expressing CD40 molecules on their surface. Nevertheless, our confocal microscopy experiments clearly demonstrated the specific binding of anti-β2GPI peptide Abs to TLR4. In fact, these autoantibodies reacted with the cell surface of HEK293T4 transfected with the TLR4 gene, but not with the wild-type HEK293 cells, and the staining completely overlapped with the signal of anti-TLR4 Abs. Because of the lack of CD40 expression in both wild-type HEK293 and HEK293T4 cell lines, we can completely exclude the cross-reactivity between anti-β2GPI peptide Abs and CD40.39–41 Furthermore, the phosphorylation of IRAK1, an adaptor protein involved in TLR signaling pathways, confirmed the specific TLR4 activation induced by anti-β2GPI peptide Abs in endothelial cells and monocytes.39,41–43
A previous study demonstrated the cross-reactivity between a subset of anti-transglutaminase Abs, typical serologic markers in patients with active celiac disease, with an epitope of the extracellular region of human TLR4 (aa 435-446). Similarly, these Abs binding the TLR4 induced monocyte activation.35
Aberrant activation of TLR signaling has a significant impact on the onset of autoimmunity. In particular, human TLR4 and its downstream signaling pathways may play a critical role in autoimmune diseases and constitute an attractive target for therapeutic intervention.44 Polymorphisms in TLR4 may also be important in the pathogenesis of autoimmune diseases, but their role appears controversial.45 Therefore, other factors (eg, autoantibodies) may play a role in determining susceptibility to chronic inflammatory human diseases such as SLE and APS.
TLR4 appears to be involved in in vivo experimental models26 of aPL-mediated thrombosis and in in vitro endothelial21 and monocyte22 activation. Growing evidence suggests that the endothelial cell activation that is induced by anti-β2GPI Abs needs the presence of β2GPI on the cell surface. The recent demonstration of the direct binding of β2GPI to TLR2, but not to TLR4, on the endothelial cell surface suggests that TLR4 may behave as a coreceptor of annexin A2.16,46,47 Therefore, the high-affinity interaction between β2GPI and annexin A248 on cell surface might engage TLR4, leading to the recruitment of myeloid differentiation factor 88 and NF-κB translocation.40 Our finding extends current knowledge about the relationships between anti-β2GPI Abs and TLR4 and strongly suggests an additional pathogenic mechanism for TLR4 stimulation without requiring β2GPI binding on cell surface. Our findings are consistent with previous evidence demonstrating that purified anti-β2GPI Abs are able to induce VCAM expression on endothelial cells and TNF-α release by monocytes, leading to an enhanced inflammatory response.21,22,49 In particular, there is substantial evidence that TNF-α may play a significant proinflammatory role both in APS and SLE. TNF-α is well known as one of the major triggers for the induction of tissue factor production by monocytes in APS. It has also been shown that aPLs induce TNF-α release in monocytes.22,50
In our experimental conditions, anti-β2GPI peptide Abs failed to bind plasma-purified β2GPI, either alone or in combination with cardiolipin, in an ELISA specific for APS diagnosis. We observed that anti-β2GPI peptide Abs recognized plasma-purified β2GPI exclusively under denaturing conditions.
How does β2GPI peptide become autoantigenic? According to the Swiss-Prot database, an epitope of P gingivalis RNA polymerase σ-70 factor shares a complete identity with the β2GPI peptide. Consistent with previous studies suggesting a molecular mimicry between periodontal pathogens and β2GPI,37 in the present study we found that the Ab reactivity to P gingivalis was significantly higher in patients positive for anti-β2GPI peptide Abs compared with healthy subjects. These results support the hypothesis that P gingivalis infection might be responsible for the generation of anti-β2GPI peptide Abs by molecular mimicry. Because the β2GPI peptide shares a lesser degree of homology with proteins encoded by other various infectious agents, such as human enterovirus A (EV71, EV92, and coxsackie A16), parvovirus 4, adenovirus type 2, hepatitis GB virus B, Saccharomyces cerevisiae and Candida albicans (data not shown), we cannot exclude that anti-β2GPI peptide Abs might also arise during infection with one of these agents. Therefore, this finding suggests the view that an exogenous agent(s) may trigger an autoimmune response, leading to a novel mechanism of pathogenic activation of TLR4.
Although our data show the potential pathogenic role of anti-β2GPI peptide Abs in inducing a proinflammatory microenvironment, in the present study we did not find a significant association and/or correlation between anti-β2GPI peptide Abs and disease activity or clinical manifestations in patients with APS and SLE. Because several studies have demonstrated a requirement for the TLR4 signaling cascade in the development of thrombotic events and lupus-like autoimmune disease in animal models,24,51 the inflammatory condition induced by TLR4 engagement could be a risk factor for the onset and progression of the clinical features typical of APS and SLE.
In conclusion, the main finding in the present study is that anti-β2GPI peptide Abs directed against a domain I cryptic epitope of the molecule have a peculiar role in APS and SLE because of direct binding to human TLR4. These Abs are functionally active, being able to induce the activation of endothelial cells and monocytes. Further evaluation of serum anti-β2GPI peptide Abs in a larger patient population needs to be done to clarify their role as prognostic/predictive biomarkers in APS and SLE. Increasing our understanding of the influence of anti-β2GPI peptide Abs on autoimmune disease pathogenesis, combined with the development of novel ways to target TLR activity, may provide downstream translational benefits in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof A. Puccetti for the kind gift of the HEK293T4 cell line and Dr A. Mastrantonio for the kind gift of P gingivalis samples.
This work was supported by a research grant from the Italian Ministry of Health (to E.O.).
Authorship
Contribution: T.C. designed and performed the research; C.A., A.L., and S.T. collected and analyzed the data; A.C. and F.D. performed the research; M.S. designed the research; M.P. performed the statistical analysis; F.C. and G.V. interpreted the data; A.S. wrote the manuscript; and E.O. and P.M. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paola Margutti, Department of Cell Biology and Neurosciences, Istituto Superiore di Sanità, Rome, Italy; e-mail: paola.margutti@iss.it.