To the editor:
Null mutations in leukocyte NADPH oxidase subunits gp91phox, p22phox, p67phox and p47phox are associated with a loss of superoxide-generating enzyme activity on both plasma and phagosomal membranes in chronic granulomatous disease (CGD) patients and CGD mouse models. In contrast, the phosphatidylinositol 3-phosphate (PtdIns3P)–binding p40phox subunit plays a selective role in regulating NADPH oxidase activity on PtdIns3P-enriched intracellular membranes such as phagosomes.1–6 To date, a single case of CGD due to defects in the NCF4 gene encoding p40phox has been reported.5 This patient is a compound heterozygote for mutations leading to a premature stop codon and R105A point mutation in p40phox ablating PtdIns3P binding, and presented with an inflammatory bowel disease–like illness at 3 years of age. Analysis of NADPH oxidase activity in neutrophils showed intact plasma membrane superoxide generation but a profound impairment in phagosomal superoxide elicited by serum opsonized zymosan (SOZ), serum opsonized Staphylococcus aureus, or IgG-coated beads.5 Similarly, neutrophils from p40phoxR58A/R58A mice harboring a knock-in mutation that disrupts p40phox binding to PtdIns3P exhibit normal plasma membrane NADPH oxidase activity but reduced phagosomal oxidant production elicited by IgG-opsonized beads or serum-opsonized S aureus.2,7 However, in contrast to p40phox-mutant human neutrophils, SOZ-elicited oxidant production in p40phox-mutant mouse neutrophil phagosomes was minimally affected. NADPH oxidase activity elicited by non-opsonized zymosan or by live Aspergillus fumigatus hyphae was also not impaired in p40phox-mutant mice (P. Hawkins and K. Anderson, personal communication, June 13, 20118,9 ). These findings suggested differences between mouse and human neutrophils in activation of oxidant production by fungal particles.
In this study, we further explored the importance of p40phox for regulation of NADPH oxidase activity in neutrophils stimulated with fungal particles. Neutrophils were isolated from peripheral blood of healthy donors or the patient with p40phox mutations5 in studies approved by the Indiana University School of Medicine and Washington University School of Medicine institutional review boards. Informed consent was provided according to the Declaration of Helsinki. Mouse bone marrow neutrophils were obtained from wild-type and p40phoxR58A/R58A knock-in mice.2,7 Luminol-based chemiluminescence assays were used to monitor oxidant production in response to heat-treated A fumigatus hyphal fragments, zymosan, or serum-opsonized zymosan.5,10
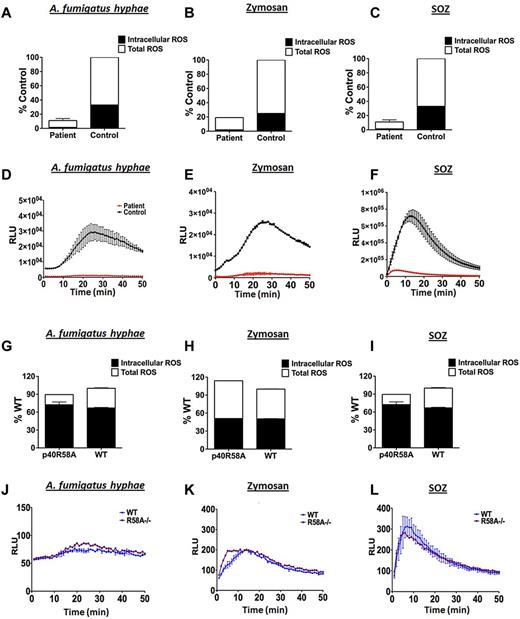
When challenged with heat-treated A fumigatus hyphae (Figure 1A,D), zymosan (Figure 1B,E) or, as previously shown,5 SOZ (Figure 1C,F), human p40phox mutant neutrophils showed a profound reduction in both total and phagosomal (intracellular) superoxide production compared to healthy donors. In striking contrast, and consistent with prior studies by Hawkins and colleagues, superoxide production in murine p40phoxR58A/R58A neutrophils stimulated with these agonists was similar to neutrophils isolated from wild-type littermate controls (Figure 1G-L).2,7–9
Oxidant production in human and murine neutrophils with defects in p40phox. Neutrophils from the patient with NCF4 mutations showed substantial decreases in both total and intracellular oxidant responses when challenged with heat-killed A fumigatus hyphal particles (A), Zymosan (B), or SOZ (C) compared to healthy controls. Contrary to human neutrophil data, p40phoxR58A/R58A mouse neutrophils show a robust total and intracellular oxidant burst to these agonists (heat-killed A fumigatus hyphae (G), Zymosan (H) and SOZ (I)). Intracellular ROS response kinetics for human neutrophils are presented in panels D through F and murine intracellular response kinetics presented in panels J through L. Oxidant production by human or murine neutrophils was determined in 96-well plates using 125μM Luminol (total extracellular and intracellular) and Luminol with superoxide dismutase (SOD; 225 U/mL; intracellular). After addition of fungal agonists, chemiluminescence was recorded over 50 minutes in a SpectrMax L Luminometer. All experiments were performed in at least duplicates (±SD). Data shown are the total integrated relative light units (RLU) expressed as % of control (N = 2-3 independent experiments) or the RLU over time from a representative experiment.
Oxidant production in human and murine neutrophils with defects in p40phox. Neutrophils from the patient with NCF4 mutations showed substantial decreases in both total and intracellular oxidant responses when challenged with heat-killed A fumigatus hyphal particles (A), Zymosan (B), or SOZ (C) compared to healthy controls. Contrary to human neutrophil data, p40phoxR58A/R58A mouse neutrophils show a robust total and intracellular oxidant burst to these agonists (heat-killed A fumigatus hyphae (G), Zymosan (H) and SOZ (I)). Intracellular ROS response kinetics for human neutrophils are presented in panels D through F and murine intracellular response kinetics presented in panels J through L. Oxidant production by human or murine neutrophils was determined in 96-well plates using 125μM Luminol (total extracellular and intracellular) and Luminol with superoxide dismutase (SOD; 225 U/mL; intracellular). After addition of fungal agonists, chemiluminescence was recorded over 50 minutes in a SpectrMax L Luminometer. All experiments were performed in at least duplicates (±SD). Data shown are the total integrated relative light units (RLU) expressed as % of control (N = 2-3 independent experiments) or the RLU over time from a representative experiment.
These results show that p40phox with an intact PtdIns3P binding domain is essential for human neutrophil oxidase activity in response to a variety of fungal particles, in contrast to murine neutrophils where it appears to be dispensable. Thus, murine p40phox deficiency may not fully model the effects in humans. There may be species differences in the receptors11,12 and/or signaling pathways stimulated by fungal cell walls that account for the differential requirement between human and mouse neutrophils for p40phox in NADPH oxidase activation. These results also suggest that humans with p40phox mutations may have impaired responses to fungal pathogens.
Authorship
Acknowledgments: The authors thank the patient and his family for their participation in this study and the staff at Alberta Children's Hospital and the National Institutes of Health Clinical Center for their care of the patient and assistance with sending blood samples. They also thank Phillip Hawkins and Len Stephens (Babraham Institute, Cambridge, United Kingdom) for providing p40phoxR58A/R58A mice.
This work was supported by the National Institutes of Health R01HL45635 (M.C.D.), the Children's Discovery Institute (M.C.D.), and the American Heart Association Midwest Affiliate Postdoctoral Fellowship (J.D.M.).
Contribution: J.B. and J.D.M. designed and performed experiments, analyzed the data, prepared the figures, and drafted the letter; A.A.A. and A.A. performed experiments; and M.C.D. oversaw the design and interpretation of the experiments, preparation of the letter and figures, and editing of the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.D.M. is Boston Children's Hospital and Harvard Medical School, Boston, MA. The current affiliation for A.A.A. is Universidad de Antioquia, Escuela de Microbiología, Medellin, Colombia.
Correspondence: Mary C. Dinauer, Department of Pediatrics, Washington University School of Medicine, 660 S Euclid, Campus Box 8208, St Louis, MO 63110; e-mail: dinauer_m@kids.wustl.edu.
References
National Institutes of Health
Author notes
J.B. and J.D.M. contributed equally to this work.