Abstract
Immediate-early host-virus interactions that occur during the first weeks after HIV infection have a major impact on disease progression. The mechanisms underlying the failure of HIV-specific CD8 T-cell response to persist and control viral replication early in infection are yet to be characterized. In this study, we performed a thorough phenotypic, gene expression and functional analysis to compare HIV-specific CD8 T cells in acutely and chronically infected subjects. We showed that HIV-specific CD8 T cells in primary infection can be distinguished by their metabolic state, rate of proliferation, and susceptibility to apoptosis. HIV-specific CD8 T cells in acute/early HIV infection secreted less IFN-γ but were more cytotoxic than their counterparts in chronic infection. Importantly, we showed that the levels of IL-7R expression and the capacity of HIV-specific CD8 T cells to secrete IL-2 on antigenic restimulation during primary infection were inversely correlated with the viral set-point. Altogether, these data suggest an altered metabolic state of HIV-specific CD8 T cells in primary infection resulting from hyperproliferation and stress induced signals, demonstrate the discordant function of HIV-specific CD8 T cells during early/acute infection, and highlight the importance of T-cell maintenance for viral control.
Introduction
Primary HIV infection is characterized by extremely high viral load with massive depletion of CD4 T cells in peripheral blood and mucosal tissues. After resolution of peak viral load, the establishment of a viral load set-point is reached when the fine balance between the efficacy of the immune control and the pathogenicity of the virus is established.1 During this chronic phase of infection, HIV-specific CD8 T cells are functionally impaired and clonally exhausted. They show a skewed maturation phenotype2,3 and express high levels of inhibitory molecules,4-6 a reduced proliferative capacity,7,8 and an increased sensitivity to Fas-induced apoptosis.9 The functional effector impairment includes loss of perforin expression,10 inability to secrete cytokines in response to antigen restimulation,11 and a reduced capacity to lyse target cells in vitro.12 The global loss of function of HIV-specific CD8 T cells in the chronic phase of infection has been well characterized in contrast to events occurring in the acute phase of infection.
Identification of persons within the first weeks of HIV infection has provided the opportunity to analyze critical events that take place in the early phase of infection. Moreover, the classification of primary HIV infection in Fiebig stages has enabled homogeneous cross-sectional studies in terms of duration of infection and primary disease stage.13 HIV-specific CD8 T cell responses emerge concomitantly in the periphery with the control of viral load and the resolution of clinical symptoms.14,15 The critical role of CD8 T cells in controlling viral replication in acute infection has been demonstrated in the SIV model,16 where CD8 T-cell depletion leads to a sharp increase in viremia. However, other studies reported that HIV-specific CD8 T cells did not reduce the life span of SIV-infected CD4 T cells and suggested that control of viral load by CD8 T cells in primary SIV infection is mediated by a noncytolytic mechanism.17 Overall, the function and mechanisms of viral control by HIV-specific CD8 T cells in primary infection have not been deciphered.18
In human subjects infected by HIV, the pressure elicited by HIV-specific CD8 T cells in vivo on viral replication is evidenced by the appearance of escape mutations in MHC class I-restricted epitopes occurring very early in HIV infection.19,20 However, the studies that analyzed the phenotypic and functional characteristics of HIV-specific CD8 T cells in acute/early infection concluded that these cells were functional in primary infection,21 and did not provide clues as to the mechanisms that lead to the lack of persistence and dysfunction of HIV-specific CD8 T cells in chronic infection. Systems-wide analysis aimed at dissecting the characteristics of HIV-specific CD8 T cells early in infection would help our understanding of the mechanisms involved in control of virus replication during primary infection and identify pathways that could predict the immune dysfunction and clonal depletion observed in chronic infection. In this study, we analyzed a primary infected cohort of 35 persons in a window of 4-10 weeks after infection and performed a thorough phenotypic, gene expression and functional analysis of the HIV-specific CD8 T cells in this acute/early phase of HIV infection and compared them with HIV-specific CD8 T cells in chronic infection as well as CMV-specific CD8 T cells as internal controls within the same donors in the 2 stages of disease.
Methods
Study participants
HIV-1–infected subjects signed informed consent forms in accordance with the Declaration of Helsinki for this study, which was approved by the Montreal University Hospital Center review board. HLA haplotype, viral load, and CD8 and CD4 T-cell counts were measured at sample collection time. Plasma viral load was measured using the Amplicor HIV-1 Monitor UltraSensitive Method (Roche Diagnostics).
Tetrameric pMHCI complexes
Soluble biotinylated pMHCI monomers were manufactured by the CANVAC tetramer core facility (Montreal, QC) as described previously22 and tetramerized with PE-conjugated extravidin (Sigma-Aldrich). For CMV, 2 pMHCI tetramers with epitopes from the pp65 protein were produced: HLA-A*0201-NLVPMVATV (NV9, 495-503) and HLA-B*0702-TPRVTGGGAM (TM10, 417-426). For HIV, the following pMHCI tetramers were produced: HLA-A*0201-FLGKIWPSHK (FK10, Gag70-79), HLA-A*0201-SLYNTVATL (SL9, Gag77-85), HLA-A*0301-RLRPGGKKK (RK9, Gag20-28), HLA-A*0301-QVPLRPMTYK (QK10, Nef73-82), HLA-B*0801-FLKEKGGL (FL8, Nef90-97), HLA-B*0801-GEIYKRWII (GI9, Gag127-135), HLA-B*0702-TPGPGVRYPL (TL10, Nef128-137), HLA-A*2301-RYPLTFGWCF (RF10, Nef134-143), and HLA-A*2401-RYPLTFGW (RW8, Nef134-141).
Autologous viral sequencing
For bulk analysis of autologous viral populations or clonal sequencing (marked with an asterisk), viral RNA was extracted using the QIAamp viral RNA minikit (QIAGEN) and reverse-transcribed using the SuperScript One-Step RT-PCR kit (Invitrogen). Amplification was performed by nested PCR using sets of outer and inner primers specific for each region of interest. Amplicons were either purified and sequenced in bulk or ligated into pGEM-T Easy vector (Promega), cloned, and sequenced.
Phenotypic analysis of antigen-specific CD8 T cells
Thawed PBMCs were stained with PE-conjugated pMHCI tetramers at 0.3 μg per 106 cells for 15 minutes at 37°C, then washed and stained for surface markers at 4°C for 20 minutes with the following monoclonal antibodies: (1) αCD3-Pacific Blue, αCCR7-PECy7, αCD27-Alexa700, αCD95-FITC, αCD45RA-APCCy7, αCD127-PECy5, αPD-1–APC (BD Biosciences), and αCD8-ECD (Beckman Coulter); (2) intracellular αKi67-FITC (BD Biosciences) staining was performed after fixation and permeabilization of cells; and (3) apoptotic cells were detected using annexin-V–FITC labeling according to the manufacturer's protocol (Bender MedSystems). Live/dead fixable Aqua (Invitrogen) was used to exclude dead cells from the analysis. Data were collected using an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (Version 8.7.3; TreeStar).
Intracellular cytokine staining assay of antigen-specific CD8 T cells
Intracellular cytokine staining assay was performed as described previously.23 Briefly, stimulated samples were prestained with the corresponding PE-conjugated pMHCI tetramers for 15 minutes at 37°C. Cells were stimulated with the relevant peptides at a concentration of 5 μg/mL for 6 hours at 37°C in the presence of brefeldinA (10 μg/mL; Sigma-Aldrich). Cells in the negative control tubes were stained with pMHCI tetramers at the end of the stimulation period. After a single wash, cells were stained with αCD3-Pacific Blue, αCD27-Pacific blue, αCD45RA-APCCy7, αCCR7-PECy7 (BD Biosciences), αCD8-ECD (Beckman Coulter) and Live/dead fixable Aqua (Invitrogen), and intracellular staining with αIL-2-FITC, αTNF-Alexa700, and αIFN-γ–APC (BD Biosciences).
Phospho-specific flow cytometric analysis of pSTAT5
Three million cells were prestained with the corresponding PE-conjugated pMHCI tetramers and Live/dead fixable Aqua (Invitrogen) for 20 minutes at room temperature. After 1 wash, cells were resuspended at 107 cells/mL in PBS and stimulated or not for 15 minutes at 37°C with IL-7 (5 ng/mL; Sigma-Aldrich). After stimulation, cells were fixed with Cytofix buffer (BD Biosciences) for 10 minutes at 37°C, then pelleted and permeabilized in PERM III buffer (BD Biosciences) for 20 minutes on ice. Cells were rehydrated in Staining Buffer (BD Biosciences) for 30 minutes on ice and stained with an antibody cocktail containing αCD3-Alexa700, αCD8-pacific blue, and αpY694Stat5-APC (BD Biosciences).
Analysis of antigen-specific CD8 T-cell cytotoxic capacity
The cytotoxic capacity of HIV- and CMV-specific CD8 T cells was measured in lytic units using the protocol detailed in Mbitikon-Kobo et al.24 Briefly, autologous B cells were positively isolated and stained with low and high concentrations of CFSE. Cells stained with the low concentration of CFSE were loaded with the cognate peptide for 1 hour at 37°C, washed twice, and mixed with cells stained with the high concentration of CFSE. The mixed B cells were incubated for 6 hours with negatively enriched CD8 T cells at different effector/target ratio (E/T). After incubation, cells were stained with the corresponding tetramer and Live/dead fixable FarRed (Invitrogen). Lytic units were calculated based on the E/T30 ratio derived from the curve of specific lysis in function of E/T ratio.
Plasmatic IL-7 quantification
Plasma IL-7 levels were determined in duplicate by an ultrasensitive immunoassay (Quantikine HS IL-7 immunoassay Kit, R&D Systems) according to the manufacturer's instructions.
Gene array and pathway analysis of significantly regulated genes
The HIV- and CMV-specific CD8 T cells that were sorted to perform the gene expression analysis are highlighted in pink in supplemental Tables 1 and 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Total RNA was purified, amplified, and hybridized onto Illumina Human Ref-8 V3 BeadChips as described previously.25 Gene expression data were analyzed using Bioconductor and filtered; of the 24 526 initial probe set, 10 630 probes were left after the filtering steps. The resulting matrix of filtered genes was generated by log2 transformation and used as input for linear modeling. The Bioconductor's limma package was used at this step to estimate the fold change between groups of HIV-specific CD8 T cells in primary and chronic infection and CMV-specific CD8 T cells by fitting a linear model and using an empirical Bayes method to moderate SEs of the estimated log-fold changes for each gene expression values. P values from the resulting comparison were adjusted for multiple testing according to the method of Benjamini and Hochberg.26 Determination of regulated gene expression was based solely on the nominal P values. Ingenuity Pathways Analysis (Ingenuity Systems, www.ingenuity.com) was used to identify the biologic functions, canonical pathways, and transcription factor regulation using the genes from the dataset that met the P value cutoff of .05.
Statistical analysis
Statistical analysis was performed using the Spearman rank for correlations and the 2-tailed Student paired or unpaired t test for group comparisons. For correlations, P values were indicated. For groups comparison, P < .05 was considered significant.
Results
Study population
We selected 35 subjects in the first 10 weeks of HIV infection called acute/early infection (AEI) with a mean of 53 days after infection (supplemental Table 1). Most subjects were sampled in Fiebig stage V, with some donors recruited in Fiebig stage IV, when HIV-specific CD8 T cells are detected in the periphery as peak viral load decreases as illustrated by the inverse correlation with the days after infection (Figure 1A).14,15 To compare the acute/early group with chronically infected donors, 23 additional untreated viremic subjects infected for > 6 months and displaying high viral loads were selected (supplemental Table 2). The mean viral load was significantly higher in the acute/early group compared with the chronically infected group (1 244 791 and 105 696 copies/mL, respectively; Figure 1B). Both groups had comparable CD4 T-cell counts, but acute/early infected subjects showed higher absolute numbers of CD8 T cells (Figure 1C). Twelve subjects recruited in the acute/early phase of infection and who did not receive antiretroviral therapy were followed longitudinally.
Phenotype of total CD8 T cells and HIV- and CMV-specific CD8 T cells during primary infection. (A) Inverse correlation between the viral load in primary HIV infection and days after infection in the studied cohort. (B) Viral load (copies/mL) in the primary and chronic studied cohorts. (C) CD4 and CD8 counts in the primary and chronic studied cohorts. (D) Percentage of HIV- or CMV-specific CD8 T cells tetramer+ in primary and chronic infection. No significant difference was observed between the percentage of HIV or CMV tetramer+ in primary infection (HIV P or CMV P) and the percentage of HIV or CMV tetramer+ in chronic infection (HIV C or CMV C). Diamonds and circles represent single specificities for HIV or CMV-tetramer+ cells, respectively; filled and open symbols represent tetramer+ cells during primary or chronic HIV infection, respectively. (E) Phenotypic analysis of HIV- and CMV-specific CD8 T cells in primary infection by multiparametric flow cytometry for the markers CCR7, CD45RA, CD27, CD127, and CD95. Diamonds and circles represent the percentage of each marker at the surface of HIV- or CMV-specific CD8 T cells, respectively. (F) Percentage of CD27 expression on HIV- or CMV-specific CD8 T cells in primary or chronic infection. HIV-specific CD8 T cells in primary infection (HIV P) express significantly higher levels of CD27 compared with HIV-specific CD8 T cells in chronic infection (HIV C) or CMV-specific CD8 T cells in primary (CMV P) or chronic infection (CMV C). (G) Heatmap of the 258 genes with P < .01 in supervised analysis between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively). Color scale represents the z-score of the expression value for each probe. (H) Heatmaps of genes significantly modulated between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively) in the protein ubiquitination and mTOR signaling pathways. Color scale represents the z-score of the mean expression value of each group for the genes significantly modulated within the pathways. **P < .005. ***P < .0005. ns indicates not significant.
Phenotype of total CD8 T cells and HIV- and CMV-specific CD8 T cells during primary infection. (A) Inverse correlation between the viral load in primary HIV infection and days after infection in the studied cohort. (B) Viral load (copies/mL) in the primary and chronic studied cohorts. (C) CD4 and CD8 counts in the primary and chronic studied cohorts. (D) Percentage of HIV- or CMV-specific CD8 T cells tetramer+ in primary and chronic infection. No significant difference was observed between the percentage of HIV or CMV tetramer+ in primary infection (HIV P or CMV P) and the percentage of HIV or CMV tetramer+ in chronic infection (HIV C or CMV C). Diamonds and circles represent single specificities for HIV or CMV-tetramer+ cells, respectively; filled and open symbols represent tetramer+ cells during primary or chronic HIV infection, respectively. (E) Phenotypic analysis of HIV- and CMV-specific CD8 T cells in primary infection by multiparametric flow cytometry for the markers CCR7, CD45RA, CD27, CD127, and CD95. Diamonds and circles represent the percentage of each marker at the surface of HIV- or CMV-specific CD8 T cells, respectively. (F) Percentage of CD27 expression on HIV- or CMV-specific CD8 T cells in primary or chronic infection. HIV-specific CD8 T cells in primary infection (HIV P) express significantly higher levels of CD27 compared with HIV-specific CD8 T cells in chronic infection (HIV C) or CMV-specific CD8 T cells in primary (CMV P) or chronic infection (CMV C). (G) Heatmap of the 258 genes with P < .01 in supervised analysis between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively). Color scale represents the z-score of the expression value for each probe. (H) Heatmaps of genes significantly modulated between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively) in the protein ubiquitination and mTOR signaling pathways. Color scale represents the z-score of the mean expression value of each group for the genes significantly modulated within the pathways. **P < .005. ***P < .0005. ns indicates not significant.
Phenotype and gene expression profile of HIV-specific CD8 T cells in primary infection
To characterize antigen-specific CD8 T cell responses, we analyzed tetramer+ cells in each subject against known immuno-dominant HIV epitopes comprising 8 HIV specificities restricted by HLA-A*0201, A*0301, B*0702, or B*0801, and 2 CMVpp65 epitopes restricted by HLA-A*0201 or HLA-B*0702 as controls (supplemental Tables 1-2). The choice of tetramers analyzed was made through the systematic screening of PBMCs for positive responses. To address the potential bias induced by epitope variation, we sequenced the autologous studied epitopes. The vast majority of primary and chronic HIV-specific CD8 T cell responses were directed against the wild type antigen (supplemental Tables 1-2). The mean frequencies of both HIV and CMV- tetramer+ CD8 T cells were comparable in primary and chronic infection (Figure 1D). The phenotype frequencies of tetramer+ assessed by multiparametric flow cytometric analysis showed that HIV-specific CD8 T cells during primary infection exhibited a skewed phenotype previously described in chronically infected subjects as predominantly CCR7−, CD45RA−, CD27high, CD127low, and CD95high (Figure 1E).2,3 This phenotype was in sharp contrast with the phenotype frequencies of control CMV-specific CD8 T cells in the same subjects, as these exhibited a terminally differentiated stage: CCR7−, CD45RA+/−, CD27low, CD127int, and CD95low (Figure 1E). Higher expression of the costimulatory molecule CD27 was the only significant difference in phenotype frequency we observed between primary and chronic infection (75.3% and 59.6%, respectively; Figure 1F). Altogether, these results demonstrated that the differentiation phenotype of HIV-specific CD8 T cells from acutely and chronically infected subjects were indistinguishable, with the exception of a slightly higher level of CD27 expression during primary infection.
To gain further insight into possible differences in the characteristics of HIV-specific CD8 T cells during the primary and chronic phase of the disease, we determined the global transcriptional profiles of these cells. We performed gene array analysis on 7 HIV-specific CD8 T cells from 5 subjects in acute/early infection, 6 HIV-specific CD8 T cells, and 4 CMV-specific CD8 T cells from 5 subjects in chronic HIV infection selected to be representative of the 2 groups as detailed in supplemental Table 3. The complete list of genes modulated in HIV-specific CD8 T cells from primary compared with HIV- or CMV-specific CD8 T cells from chronic HIV infection is presented in supplemental Table 4. The top 258 significant genes (P < .01) represented in the heatmap of supervised cluster analysis discriminated HIV-specific CD8 T cells in acute/early infection from HIV- and CMV-specific CD8 T cells in chronic infection (Figure 1G). We then subjected these genes to pathway analysis to identify biologic functions and processes significantly modulated in HIV-specific CD8 T cells in acute/early infection. This analysis revealed that HIV-specific CD8 T cells during primary infection exhibit distinct metabolic processes compared with chronic infection as exemplified by the mTOR signaling pathway as well as protein ubiquitination pathway; both pathways central for regulating cellular processes were significantly modulated in HIV-specific CD8 T cells in primary infection compared with HIV- and CMV-specific CD8 T cells in chronic infection (Figure 1H). Thus, although HIV-specific CD8 T cells in acute/early infection exhibited a phenotype comparable to HIV-specific CD8 T cells in chronic infection, they have a distinct metabolic state compatible with high levels of activation and proliferation.
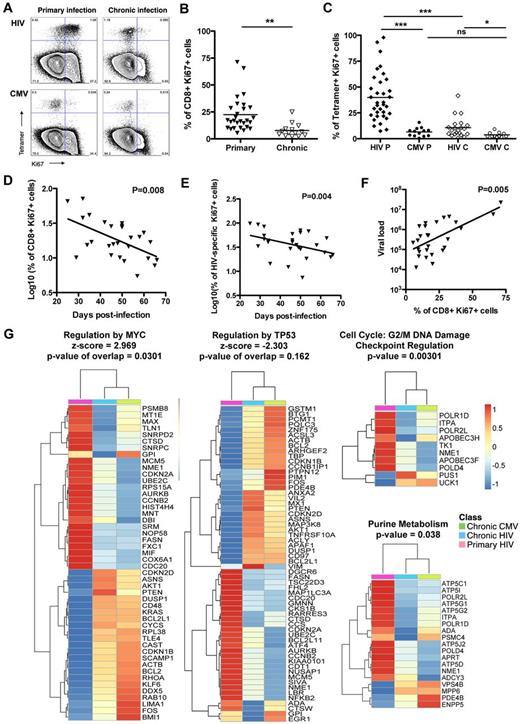
High levels of proliferation of HIV-specific CD8 T cells in primary infection
To show the heightened proliferative state of the HIV-specific CD8 T cells in acute/early HIV infection, we measured the expression of the nuclear marker Ki67 on total CD8 T cells and HIV- and CMV-specific CD8 T cells. A significantly higher frequency of Ki67+ was measured on total CD8 T cells during primary infection compared with chronic infection (22.4% and 7.8%, respectively; Figure 2A-B), and on HIV-specific CD8 T cells in primary infection compared with chronic infection (39.9% and 10.7%, respectively; Figure 2A,C). When compared with HIV-specific CD8 T cells, the frequencies of CMV-specific CD8 T cells expressing Ki67 were significantly lower than that observed for HIV specific T cells at both stages of the disease (6.5% and 3.7%; Figure 2A,C). Furthermore, in acutely infected subjects, the percentages of Ki67+ cells on both total CD8 T cells and HIV-specific CD8 T cells were inversely correlated with days after infection (Figure 2D-E). Of note, Ki67 expression on total CD8 T cells was positively correlated with viral load (Figure 2F). In line with these results, the gene expression analysis revealed that the regulation of target genes downstream of the transcription factors controlling cell cycle and cell proliferation were also significantly modulated in HIV-specific CD8 T cells in acute/early infection compared with HIV- or CMV-specific CD8 T cells in chronic infection: activated regulation by MYC (z-score = 2.969) and inhibited regulation by TP53 (z-score = -2.303; Figure 2G). Furthermore, the G2/M DNA damage checkpoint and the purine metabolism pathways were significantly up-regulated in HIV-specific CD8 T cells from acutely infected subjects (Figure 2G). The modulations of these pathways suggests that HIV-specific CD8 T cells are not only entering into cycle as shown by the Ki67 staining but also undergo mitosis and continual progression through the cell cycle. These heightened levels of proliferation are most probably responsible for the significantly higher absolute number of CD8 T cells in primary infection compared with chronic infection (supplemental Figure 1A). Thus, these data suggest that HIV-specific CD8 T cells are proliferating extensively in vivo in the acute/early phase of infection.
Proliferation of total CD8 T cells and HIV- and CMV-specific CD8 T cells in primary infection. (A) Dot plots of Ki67 expression of HIV- and CMV-specific CD8 T-cell responses for subject AEI 333 in primary and chronic infection. (B) Percentage of Ki67 expression on CD8 T cells in primary and chronic infection. Total CD8 T cells exhibit significantly higher levels of Ki67 in primary infection compared with chronic infection. Filled and open symbols represent CD8 T cells studied in primary and chronic HIV infection, respectively. (C) Expression of Ki67 on HIV- and CMV-specific CD8 T cells tetramer+ (diamonds and circles, respectively) in primary and chronic infection (filled and open symbols, respectively). HIV-specific CD8 T cells in primary infection (HIV P) express significantly higher levels of Ki67 compared with HIV-specific CD8 T cells in chronic infection (HIV C) or CMV-specific CD8 T cells in primary (CMV P) or chronic infection (CMV C). (D) Inverse correlation between the percentage of total CD8 T cells expressing Ki67 and the days after infection. (E) Inverse correlation between the percentage of Ki67+ HIV-specific CD8 T cells and the days after infection. (F) Correlation between the percentage of Ki67 expression on total CD8 T cells and the viral load in primary infection. (G) Heatmaps of genes significantly modulated between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively) in cell cycle pathways. Color scale represents the z-score of the mean expression value of each group for the genes significantly modulated within the pathways. *P < .05. **P < .005. ***P < .0005. ns indicates not significant.
Proliferation of total CD8 T cells and HIV- and CMV-specific CD8 T cells in primary infection. (A) Dot plots of Ki67 expression of HIV- and CMV-specific CD8 T-cell responses for subject AEI 333 in primary and chronic infection. (B) Percentage of Ki67 expression on CD8 T cells in primary and chronic infection. Total CD8 T cells exhibit significantly higher levels of Ki67 in primary infection compared with chronic infection. Filled and open symbols represent CD8 T cells studied in primary and chronic HIV infection, respectively. (C) Expression of Ki67 on HIV- and CMV-specific CD8 T cells tetramer+ (diamonds and circles, respectively) in primary and chronic infection (filled and open symbols, respectively). HIV-specific CD8 T cells in primary infection (HIV P) express significantly higher levels of Ki67 compared with HIV-specific CD8 T cells in chronic infection (HIV C) or CMV-specific CD8 T cells in primary (CMV P) or chronic infection (CMV C). (D) Inverse correlation between the percentage of total CD8 T cells expressing Ki67 and the days after infection. (E) Inverse correlation between the percentage of Ki67+ HIV-specific CD8 T cells and the days after infection. (F) Correlation between the percentage of Ki67 expression on total CD8 T cells and the viral load in primary infection. (G) Heatmaps of genes significantly modulated between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively) in cell cycle pathways. Color scale represents the z-score of the mean expression value of each group for the genes significantly modulated within the pathways. *P < .05. **P < .005. ***P < .0005. ns indicates not significant.
Lower survival capacity of HIV-specific CD8 T cells in primary infection
We next aimed at assessing whether proliferating cells from acutely infected subjects were more susceptible to spontaneous apoptosis, which could explain their lack of persistence. We measured annexin-V staining in total CD8 T cells and HIV- and CMV-specific CD8 T cells after an overnight resting of the PBMCs in vitro. Total CD8 T cells were significantly more prone to apoptosis in acute/early infection compared with chronic infection with a mean of 35.8% and 16.8% annexin-V positive cells, respectively (Figure 3A-B). The frequency (64.4%) of HIV-specific CD8 T cells positive for annexin-V was significantly higher in primary infection compared with HIV-specific CD8 T cells from chronically infected subjects that showed a frequency of annexin-V+ cells (38.4%) comparable with CMV-specific CD8 T cells in acute/early infection (Figure 3A,C). In agreement with these findings, pathways involved in mitochondrial dysfunction and oxidative stress were significantly up-regulated in HIV-specific CD8 T cells in primary HIV infection compared with HIV- or CMV-specific CD8 T cells in chronic infection (Figure 3D), whereas the NRF2-mediated oxidative stress response pathway, involved in degrading oxidized proteins and relieving oxidative stress was significantly down-regulated (Figure 3D). These data suggest that HIV-specific CD8 T cells in acute/early infection fail to generate the biosynthetic and energy requirements needed for maintained proliferation, leading to increased mitochondrial dysfunction and an enhanced susceptibility to apoptosis.27
Susceptibility to spontaneous apoptosis of HIV- and CMV-specific CD8 T cells in primary infection. (A) Dot plots for annexin-V staining of HIV-specific CD8 T cells and total CD8 T cells for subject AEI 041 in primary and chronic infection. (B) Total CD8 T cells have a significantly higher percentage of annexin-V+ cells in primary infection compared with chronic infection. (C) Percentage of annexin-V+ tetramer+ cells for HIV- and CMV-specific CD8 T cells (diamonds and circles, respectively) in primary and chronic HIV infection (filled and open symbols, respectively). HIV-specific CD8 T cells in primary infection (HIV P) express higher levels of annexin-V compared with HIV-specific CD8 T cells in chronic infection (HIV C) or CMV-specific CD8 T cells in primary or chronic infection (CMV P and CMV C, respectively). (D) Heatmaps of genes significantly modulated between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively) in pathways of mitochondrial function and oxidative stress. Color scale represents the z-score of the mean expression value of each group for the genes significantly modulated within the pathways. ***P < .0005. ns indicates not significant.
Susceptibility to spontaneous apoptosis of HIV- and CMV-specific CD8 T cells in primary infection. (A) Dot plots for annexin-V staining of HIV-specific CD8 T cells and total CD8 T cells for subject AEI 041 in primary and chronic infection. (B) Total CD8 T cells have a significantly higher percentage of annexin-V+ cells in primary infection compared with chronic infection. (C) Percentage of annexin-V+ tetramer+ cells for HIV- and CMV-specific CD8 T cells (diamonds and circles, respectively) in primary and chronic HIV infection (filled and open symbols, respectively). HIV-specific CD8 T cells in primary infection (HIV P) express higher levels of annexin-V compared with HIV-specific CD8 T cells in chronic infection (HIV C) or CMV-specific CD8 T cells in primary or chronic infection (CMV P and CMV C, respectively). (D) Heatmaps of genes significantly modulated between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively) in pathways of mitochondrial function and oxidative stress. Color scale represents the z-score of the mean expression value of each group for the genes significantly modulated within the pathways. ***P < .0005. ns indicates not significant.
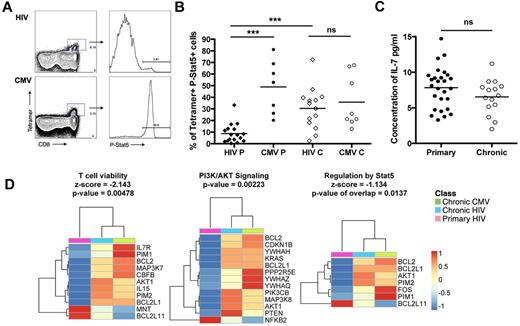
As IL-7 has been shown to be required for the survival of CD8 T cells,28 the response to IL-7 was measured by stimulating PBMCs with 5 ng/mL of IL-7 (saturating concentration; data not shown) for 15 minutes at 37°C. No difference in Stat5 phosphorylation was observed on total CD8 T cells between primary and chronic infection (data not shown). However, HIV-specific CD8 T cells phosphorylated Stat-5 at a significantly lower level in primary infection compared with HIV-specific CD8 T cells in primary infection (30.4%; Figure 4A-B) and compared with CMV-specific CD8 T cells in chronic infection (8.7% and 48.9%, respectively). This lack of IL-7 responsiveness was not the result of differences in plasma IL-7, as plasma levels were similar during primary and chronic infection (7.8 pg/mL and 6.5 pg/mL, respectively; Figure 4C). Accordingly, our gene array analysis showed a significant down-regulation of the pathways involved in T-cell viability (z-score = −2.143), PI3K/AKT signaling, and regulation by STAT5 (z-score = −1.134; Figure 4D), suggesting that HIV-specific CD8 T cells in primary infection are short lived are not responsive to either IL-7 or IL-15 signals during the hyperproliferative phase.29 The unresponsiveness to IL-7 could be attributed to a lower level of newly produced IL-7R on HIV-specific CD8 T cells in primary compared with chronic HIV infection as we observed a fold decrease (−1.8) in CD127 expression in HIV-specific CD8 T cells in acute/early infection compared with chronic (supplemental Table 3). Altogether, these data suggest that HIV-specific CD8 T cells have an impaired capacity to survive in acute/early infection because of mitochondrial dysfunction and impaired γC receptor cytokine responsiveness.
Phosphorylation of Stat-5 in response to exogenous IL-7 for HIV- and CMV-specific CD8 T cells in primary infection. (A) Dot plots for P-Stat5 staining on subjects AEI049 V2 and AEI003622 V2 in primary infection for B8FL8 and B7TM10-specific responses, respectively. (B) Percentage of P-Stat5 after 15 minutes with IL-7 at 5 ng/mL on tetramer+ cells for HIV (diamond) and CMV (circles)-specific CD8 T cells in primary and chronic infection. (C) Concentration of IL-7 (pg/mL) in plasma of patients in primary infection compared with chronic infection. (D) Heatmaps of genes significantly modulated between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively) in the γC receptor cytokine signaling pathways. Color scale represents the z-score of the mean expression value of each group for the genes significantly modulated within the pathways. ***P < .0005. ns indicates not significant.
Phosphorylation of Stat-5 in response to exogenous IL-7 for HIV- and CMV-specific CD8 T cells in primary infection. (A) Dot plots for P-Stat5 staining on subjects AEI049 V2 and AEI003622 V2 in primary infection for B8FL8 and B7TM10-specific responses, respectively. (B) Percentage of P-Stat5 after 15 minutes with IL-7 at 5 ng/mL on tetramer+ cells for HIV (diamond) and CMV (circles)-specific CD8 T cells in primary and chronic infection. (C) Concentration of IL-7 (pg/mL) in plasma of patients in primary infection compared with chronic infection. (D) Heatmaps of genes significantly modulated between HIV-specific CD8 T cells in primary infection (pink) and HIV- and CMV-specific CD8 T cells in chronic infection (blue and green, respectively) in the γC receptor cytokine signaling pathways. Color scale represents the z-score of the mean expression value of each group for the genes significantly modulated within the pathways. ***P < .0005. ns indicates not significant.
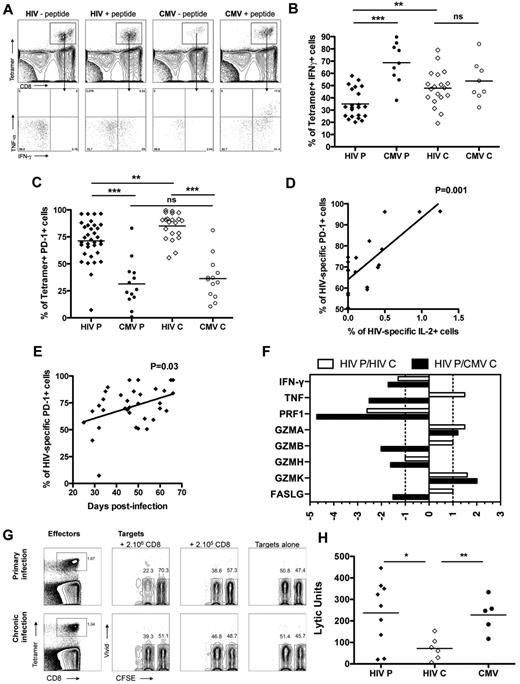
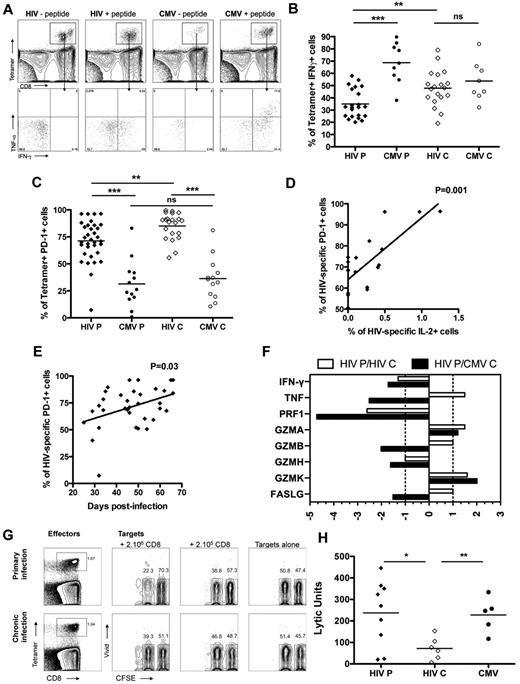
Discordant cytokine production and cytolytic capacity of HIV-specific CD8 T cells in primary infection
To assess the functional capacity of HIV-specific CD8 T cells to secrete cytokines in primary infection, we measured cytokine secretion after 6 hours of stimulation with the cognate peptide by intracellular staining on tetramer+ cells. HIV-specific CD8 T cells in acute/early infection produced significantly less IFN-γ compared with CMV-specific CD8 T cells in primary and chronic infection with means of 34.9% and 68.7%, respectively, but also significantly less IFN-γ compared with HIV-specific CD8 T cells in chronic infection (47.8%; Figure 5A-B) with comparable frequencies of tetramer+ cells (supplemental Figure 1C). Of note, HIV-specific CD8 T cells in primary and chronic infection were also secreting very limited amounts of TNF and IL-2 compared with CMV-specific CD8 T cells (supplemental Figure 1A-B), indicating that the lack of polyfunctionality observed in chronic HIV infection was already evident in primary infection. We measured the expression of activation/coinhibitory receptor PD-1 on HIV-specific CD8 T cells to assess the role of PD-1 in immune dysfunction during acute/early infection. No difference was seen in PD-1 expression on total CD8 T cells between primary and chronic infection (data not shown). PD-1 expression was higher on HIV-specific CD8 T cells both in primary and chronic infection compared with CMV-specific CD8 T cells but was significantly lower on HIV-specific CD8 T cells in primary infection compared with HIV-specific CD8 T cells in chronic infection with means of 71.2% and 85%, respectively (Figure 5C). The percentage of PD-1+ HIV-specific CD8 T cells was positively correlated with days after infection in acute/early HIV infection, suggesting an increase in the frequency of cells expressing PD-1 with time after infection (Figure 5D). Moreover, we observed a positive correlation between the frequency of HIV-specific CD8 T cells expressing PD-1 and the percentage of IL-2 production by these cells in primary infection (Figure 5E), invoking PD-1's role as a sensitive activation marker at this stage of infection.30
Functionality of HIV- and CMV-specific CD8 T cells in primary infection. (A) Dot plot for intracellular cytokine staining on tetramer+ cells on subject AEI041 V1 in primary infection for B8FL8 and B7TM10 specific responses. TNF and IFN-γ were measured within the tetramer+ cells. (B) Percentage of IFN-γ-secreting cells in tetramer+ cells for HIV (diamond) and CMV (circles) specific CD8 T cells in primary and chronic infection. (C) Expression of PD-1 on tetramer+ cells for HIV (diamond) and CMV (circles) specific CD8 T cells in primary and chronic infection. (D) Correlation between the percentage of PD-1+ cells on HIV-specific CD8 T cells and the days after infection. (E) Correlation between the percentage of PD-1+ cells on HIV-specific CD8 T cells in primary infection and the percentage of these cells expressing IL-2 after antigenic restimulation. (F) Differential gene expression in HIV-specific CD8 T cells in primary HIV infection relative to HIV- or CMV-specific CD8 T cells in chronic infection (white and gray bars, respectively). (G) Dot plots for cytotoxic activity assay on subject AEI011 in primary infection and subject AEI42117 in chronic infection for B8FL8-specific responses. B8FL8 tetramer+ cells are represented as effector cells, and CFSE-labeled autologous B cells are represented as target cells. Mixed CFSE high (nonloaded) and CFSE low B cells (loaded with the FL8 peptide) are incubated in the presence or absence of different numbers of autologous CD8 T cells. (H) Lytic units of tetramer+ cells for HIV (diamond) and CMV (circles) specific CD8 T cells in primary and chronic infection. *P < .05. **P < .005. ***P < .0005. ns indicates not significant.
Functionality of HIV- and CMV-specific CD8 T cells in primary infection. (A) Dot plot for intracellular cytokine staining on tetramer+ cells on subject AEI041 V1 in primary infection for B8FL8 and B7TM10 specific responses. TNF and IFN-γ were measured within the tetramer+ cells. (B) Percentage of IFN-γ-secreting cells in tetramer+ cells for HIV (diamond) and CMV (circles) specific CD8 T cells in primary and chronic infection. (C) Expression of PD-1 on tetramer+ cells for HIV (diamond) and CMV (circles) specific CD8 T cells in primary and chronic infection. (D) Correlation between the percentage of PD-1+ cells on HIV-specific CD8 T cells and the days after infection. (E) Correlation between the percentage of PD-1+ cells on HIV-specific CD8 T cells in primary infection and the percentage of these cells expressing IL-2 after antigenic restimulation. (F) Differential gene expression in HIV-specific CD8 T cells in primary HIV infection relative to HIV- or CMV-specific CD8 T cells in chronic infection (white and gray bars, respectively). (G) Dot plots for cytotoxic activity assay on subject AEI011 in primary infection and subject AEI42117 in chronic infection for B8FL8-specific responses. B8FL8 tetramer+ cells are represented as effector cells, and CFSE-labeled autologous B cells are represented as target cells. Mixed CFSE high (nonloaded) and CFSE low B cells (loaded with the FL8 peptide) are incubated in the presence or absence of different numbers of autologous CD8 T cells. (H) Lytic units of tetramer+ cells for HIV (diamond) and CMV (circles) specific CD8 T cells in primary and chronic infection. *P < .05. **P < .005. ***P < .0005. ns indicates not significant.
To assess the in vivo cytolytic capacity of HIV-specific CD8 T cells in acute/early infection, we first analyzed gene expression of key cytolytic effector molecules (supplemental Table 4). HIV-specific CD8 T cells expressed lower levels of IFN-γ, perforin, granzyme B, granzyme H, and FasL in primary infection compared with HIV- and CMV-specific CD8 T cells in chronic infection. However, HIV-specific CD8 T cells expressed higher levels of TNF, granzyme A, and granzyme K compared with HIV-specific CD8 T cells in chronic infection (Figure 5F), suggesting that a different mechanism of cytolytic activity may be operative. To determine the effectiveness of HIV-specific CD8 T cells from acute/early infection to lyse infected target cells, we measured the intrinsic killing capacity of HIV-specific CD8 T cells in a new assay to quantify killing in lytic units (Figure 5G).24 We observed that HIV-specific CD8 T cells in primary infection exhibited a significantly higher cytotoxic capacity than HIV-specific CD8 T cells in chronic infection (mean lytic units of 237 and 72, respectively) and comparable with the cytotoxic capacity of CMV-specific CD8 T cells (mean lytic unit of 227; Figure 5H) with comparable frequencies of tetramer+ cells (supplemental Figure 1D). Thus, despite an impaired capacity to secrete IFN-γ after antigenic restimulation and low expression of conventional lytic molecules perforin and granzyme, HIV-specific CD8 T cells in acute/early infection showed an enhanced cytotoxic capacity mediated by an unorthodox mechanism of cytotoxicity than the one used by CD8 T cells during chronic infection.
Characteristics of HIV-specific CD8 T cells in primary infection that correlates with viral control in chronic infection
In the group of subjects that did not receive antiretroviral therapy in the acute/early phase of infection and were followed longitudinally, we were able to analyze the measured parameters that would correlate with viral control. The changes observed in the cross-sectional study were confirmed in a longitudinal analysis of this group of subjects: HIV-specific CD8 T cells in the primary phase of infection were detected at similar frequencies compared with the chronic phase (Figure 6A); however, they expressed significantly higher levels of Ki67 and lower levels of PD-1 and CD127 (Figure 6B-D). They also demonstrated a decreased capacity to phosphorylate Stat5 in response to IL-7 and significantly lower levels of IFN-γ secretion on antigenic restimulation (Figure 6E-F). Among all parameters measured on HIV-specific CD8 T cells in this study, only 2 correlated with the viral load set-point. The expression of IL-7R on HIV-specific CD8 T cells in primary infection was inversely correlated with the viral load set-point (P = .005; Figure 6G). In line with this observation, the capacity of HIV-specific CD8 T cells to secrete IL-2 in primary infection inversely correlated with the viral load set-point (Figure 6H), suggesting that preservation of the capacity for autocrine IL-2 production could sustain proliferation of HIV-specific CD8 T cells and would lead to better control of viral replication. These data suggest that survival of HIV-specific CD8 T cells in primary infection could result in a better control of viral replication during chronic infection.
HIV-specific CD8 T-cell parameters in primary HIV infection that correlate with viral control in chronic infection. (A) Comparable frequencies of tetramer+ cells from the primary to chronic phase of infection in the same donors. Differential expression of Ki67 (B), PD-1 (C), and CD127 (D) by HIV-specific CD8 T cells between the acute/early and chronic time points. Longitudinal analysis of the Stat5 phosphorylation (E) and the secretion of IFN-γ (F) by HIV-specific CD8 T cells. (G) Inverse correlation between the viral load set-point and the expression of CD127 on HIV-specific CD8 T cells in primary infection. (H) Inverse correlation between the percentage of HIV-specific CD8 T cells in primary infection expressing IL-2 in response to antigen and the viral load set-point. *P < .05. **P < .005. ***P < .0005. ns indicates not significant.
HIV-specific CD8 T-cell parameters in primary HIV infection that correlate with viral control in chronic infection. (A) Comparable frequencies of tetramer+ cells from the primary to chronic phase of infection in the same donors. Differential expression of Ki67 (B), PD-1 (C), and CD127 (D) by HIV-specific CD8 T cells between the acute/early and chronic time points. Longitudinal analysis of the Stat5 phosphorylation (E) and the secretion of IFN-γ (F) by HIV-specific CD8 T cells. (G) Inverse correlation between the viral load set-point and the expression of CD127 on HIV-specific CD8 T cells in primary infection. (H) Inverse correlation between the percentage of HIV-specific CD8 T cells in primary infection expressing IL-2 in response to antigen and the viral load set-point. *P < .05. **P < .005. ***P < .0005. ns indicates not significant.
Discussion
In this study, we performed extensive analysis of the gene expression, phenotype, and function of HIV-specific CD8 T cells in acutely infected persons in an attempt to define the mechanisms that lead to the lack of persistence and dysfunction of HIV-specific CD8 T cells in chronic infection. We identified distinct metabolic states and differences in survival and functional properties between HIV-specific CD8 T cells in primary versus chronic infection, although these cells expressed the same skewed phenotype (CCR7−/CD45RA−/CD27+) previously described in chronic infection. We demonstrated here that HIV-specific CD8 T cells display different survival and functional programs while they exhibit the same differentiation phenotype early and late in HIV infection. Our data confirm previous reports showing that HIV-specific and total CD8 T-cell responses in acutely HIV-infected persons are highly activated, proliferate extensively, and are more susceptible to apoptosis.3,8,31 The survival defect and the skewed phenotype established early within the first few weeks of infection have also been described in the SIV model.32 We showed that HIV-specific CD8 T cells express lower levels of IL-7R in acute/early infection compared with chronic infection and are not responding to survival signals through the phosphorylation of Stat5. Interestingly, we demonstrated here that this susceptibility to apoptosis is associated with a metabolic state where these cells exhaust their mitochondria to sustain their proliferation. This hyperproliferation and altered mitochondrial state could lead to premature T-cell death, as it has been described in systemic lupus erythematosus.27 This apoptosis could contribute to the clonal depletion described in previous studies.33,34 Our results are consistent with recent findings that show that antigen-activated CD8 cells that receive sustained activation signals through TCR and inflammatory cytokines are driven to a hyperproliferative state associated with increased metabolic stressors, including the generation of reactive oxygen species, hypoxia, and a DNA damage response.35
A few reports have suggested that HIV-specific CD8 T cells possess weak cytolytic and effector activity in primary HIV infection by analyzing their cytokine secretion after peptide restimulation or their perforin content.36,37 In accordance with previous studies, we showed here that HIV-specific CD8 T cells in acute infection express low levels of perforin and were mostly monofunctional.38 In the LCMV model, virus-specific CD8 T cells lose progressively their capacity to secrete IL-2, TNF, and IFN-γ over time from the acute to the chronic phase of infection.39 In contrast, we showed that HIV-specific CD8 T cells in acute/early infection have an impaired capacity to secrete IFN-γ, both in vivo and after antigenic restimulation ex vivo compared with chronic infection. As HIV-specific CD8 T cells have an impaired capacity to secrete IFN-γ in primary infection, the use of this readout to quantify the frequency of HIV-specific CD8 T cells in this phase of infection might not be optimal as it would underestimate the number of HIV-specific CD8 T cells. One could argue that the viral load in our cohort of acute/early infected subjects is higher than the group of chronically infected persons, which could explain the lower levels of IFN-γ secretion.40 However, when selecting the subjects to have a similar mean viral loads in both groups, the secretion of IFN-γ by HIV-specific CD8 T cells was still significantly lower in primary infection compared with chronic infection (data not shown). To address the potential bias induced by epitope variation caused by viral escape mutations, we sequenced the autologous sequences for the studied epitopes and showed that the majority of acute/early responses were directed against the wild-type antigen present in the study subjects. Moreover, these cells still express PD-1, which suggests that their TCR is still engaged. We have previously demonstrated that the loss of antigenic stimulation via escape mutation results in an increased function of HIV-specific CD8 T-cell responses directed against the wild-type epitope.23 Therefore, our data suggest that the reduced cytokine secretion observed in the acutely infected persons is not the result of inefficient TCR signaling by altered ligands.
Surprisingly, despite impairment in the ability to secrete IFN-γ, HIV-specific CD8 T cells in primary infection displayed a greater cytolytic capacity than those in chronic infection, comparable with the cytotoxic activity of CMV-specific CD8 T cells. Previous reports have shown a positive correlation between the IFN-γ secretion and the cytotoxicity of virus-specific CD8 T cells in chronic or resolved infections, such as EBV, CMV, HIV, and influenza.41 The absence of a correlation between the IFN-γ secretion and the cytotoxic capacity observed in primary HIV infection is in line with the single-cell analysis of cytokine secretion and cytolysis performed by Varadarajan et al.42 and could be explained by an alternate cytotoxic mechanism for HIV-specific CD8 T cells in acute/early infection. This hypothesis is supported by our findings of the differential expression of effector molecules observed in HIV-specific CD8 T cells in primary infection compared with chronic infection. Of note, this superior killing capacity of HIV-specific CD8 T cells in acute/early infection is in accordance with the mathematical model predicting the control of viral replication from the rate of loss of founder virus proposed by Goonetilleke et al43 as well as the potent CD8-mediated virus inhibition during acute HIV infection described by Freel et al.44
By analyzing the phenotype of total CD4 and CD8 T cells in a large cohort of early HIV infection, Ganesan et al showed that proliferation of CD4 and CD8 T cells as well as loss of CD127+ CD8 T cells were associated with disease progression.45 In this study, we showed that the only parameters of HIV-specific CD8 T cells in acute/early infection that correlated with the viral set-point were the expression of IL-7R on these cells and their capacity to secrete some IL-2 after antigenic restimulation. These results, albeit on a small number of subjects, suggest that the maintenance of T-cell memory could play an important role in the control of disease progression. In light of a recent study demonstrating the role of mitochondrial respiratory capacity in the regulation of CD8 T-cell memory development,46 our data strongly argue for a crucial role of cellular metabolic pathways in the maintenance of HIV-specific CD8 T cells and viral control. These metabolic changes might also be implicated in the maintenance of cytolytic capacity of HIV-specific CD8 T cells in contrast to the loss of cytokine production.
Some characteristics of HIV-specific CD8 T cells in acute/early infection have been described also in EBV acute infection,47 and suggest that hyperproliferation and susceptibility to apoptosis would be the hallmark of antigen-specific CD8 T cells in the acute phase of chronic infections. Reports have demonstrated that the dysfunction of HIV-specific CD8 T cells is established over time as treatment initiation during acute infection has been shown to preserve some effector functions.48,49 It will be important to compare the characteristics of HIV-specific CD8 T cells at earlier stages of the primary infection in Fiebig stages I/II to determine when the metabolic switch associated with hyperproliferation and survival defects is established in the course of early HIV infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Reseau SIDA et Maladies Infectieuses/Fonds de la Recherche en Sante du Quebec for providing the samples for this study; the subjects for their participation in this study; Mario Legault and Chantal Grignon for clinical assistance; Maryse Lainesse, Jean-Baptiste Loubert, Luciana Tonelli, Pearline Ngauv, and Remi Fromentin for technical assistance; and Jeff Ahlers for critical review of the manuscript.
This work was supported by the Canadian Institute for Health Research, the FRSQ SIDA-MI, and the Office of Tourism, Trade and Economic Development of Florida.
Authorship
Contribution: L.T. designed and performed experiments, analyzed data, designed figures, and wrote the manuscript; F.-M.M.-K., Y.P., Y.S., J.V.G., and F.A.P. performed experiments; J.-P.G. performed gene expression analysis; M.R.B. and J.-P.R. provided the subjects; and N.C., E.K.H., and R.-P.S. contributed to design of the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lydie Trautmann, Vaccine and Gene Therapy Institute-Florida, 9801 SW Discovery Way, Port Saint Lucie, FL 34987; e-mail: ltrautmann@vgtifl.org.