Abstract
We conducted a gene therapy trial in 10 patients with adenosine deaminase (ADA)–deficient severe combined immunodeficiency using 2 slightly different retroviral vectors for the transduction of patients' bone marrow CD34+ cells. Four subjects were treated without pretransplantation cytoreduction and remained on ADA enzyme-replacement therapy (ERT) throughout the procedure. Only transient (months), low-level (< 0.01%) gene marking was observed in PBMCs of 2 older subjects (15 and 20 years of age), whereas some gene marking of PBMC has persisted for the past 9 years in 2 younger subjects (4 and 6 years). Six additional subjects were treated using the same gene transfer protocol, but after withdrawal of ERT and administration of low-dose busulfan (65-90 mg/m2). Three of these remain well, off ERT (5, 4, and 3 years postprocedure), with gene marking in PBMC of 1%-10%, and ADA enzyme expression in PBMC near or in the normal range. Two subjects were restarted on ERT because of poor gene marking and immune recovery, and one had a subsequent allogeneic hematopoietic stem cell transplantation. These studies directly demonstrate the importance of providing nonmyeloablative pretransplantation conditioning to achieve therapeutic benefits with gene therapy for ADA-deficient severe combined immunodeficiency.
Introduction
Adenosine deaminase (ADA) is an enzyme involved in purine metabolism and is essential for lymphocyte development, survival, and function. ADA deficiency is an autosomal-recessive inherited disorder that can result in severe combined immunodeficiency (SCID). Affected infants with ADA-deficient SCID usually are diagnosed by 6 months of age, after presenting with failure to thrive and recurrent opportunistic infections, because of profound pan-lymphopenia and the virtual absence of humoral and cellular immunity. ADA-deficient SCID is almost always fatal by 2 years of age if immunity is not restored.1-3
The long-standing treatment of choice for ADA-deficient SCID is a hematopoietic stem cell (HSC) transplantation from an unaffected, HLA-matched sibling.4 However, in most cases, suitable HLA-matched related donors are not available. HSC transplantations from a closely HLA-matched, unrelated donor (MUD) or a haploidentical (parental) donor, via the use of T-cell depletion, may be performed. However, outcomes from MUD or haploidentical transplantations are significantly worse than those from matched siblings because of graft-versus-host disease and engraftment failure.5
An alternative, nontransplantation option is enzyme-replacement therapy (ERT) with polyethylene glycol–modified bovine adenosine deaminase (PEG-ADA), given 1-2 times per week by intramuscular injection.6 Since this therapy became available in the late 1980s, more than 150 patients have been treated worldwide.5 ERT is life-sustaining and can restore protective immune function. However, its effects may be limited, and most patients do not achieve full immune reconstitution.7 The high cost of ERT and the required life-long injections limits its access and may create severe financial burdens on patients and their families.
Because of the limitations of these conventional therapies, gene correction of autologous HSC has been explored as a potentially curative treatment for ADA-deficient SCID. The use of a patient's own HSC bypasses the need for a donor and immunologic problems associated with allogeneic transplantations. In the 1990s, clinical trials for ADA-deficient SCID were performed in Europe and in the United States with the use of γ-retroviral vector–mediated transfer of the human ADA cDNA into bone marrow or cord blood CD34+ HSC.8-10 Unfortunately, these studies achieved only low numbers of ADA-gene corrected blood cells. On the basis of ethical considerations of risk and benefit, these early trials did not involve pretransplantation cytoreductive conditioning with chemotherapy, which may aid engraftment but carries the risks of side-effects. In addition, subjects continued to receive ERT, which may have blunted the selective advantage of emerging gene-corrected T-lymphocytes. No significant clinical benefits occurred.
By the end of the 1990s, improved techniques for isolation and gene modification of human HSC fostered the implementation of new clinical trials of gene transfer to autologous HSC for treatment of ADA-deficient SCID.11-17 These trials yielded clinically beneficial immune reconstitution with marrow conditioning with busulfan or melphalan at nonmyeloablative dosages used to achieve a greater level of engrafted stem cells.
In 2001, we initiated a clinical trial using autologous bone marrow CD34+ cells with improved γ-retroviral vectors and more effective methods for gene transfer to the stem cells. In previous studies in murine models of HSC gene transfer, we demonstrated improved expression and resistance to silencing of retroviral vectors carrying the myeloproliferative sarcoma virus (MPSV) long terminal repeat (LTR) featuring specific modifications (deletion of a negative control region and alterations of the adjacent primer binding site).18-20 It was unclear, however, that such modifications would confer the same benefits on expression in human cells. Therefore, in our clinical trial we compared 2 vectors either by using the nonmodified MPSV LTR or its modified version, MND. The CD34+ cells for each subject were divided into 2 equal aliquots, separately transduced with 1 of the 2 vectors and remixed for clinical administration. Four subjects received intravenous infusion of gene-modified autologous bone marrow CD34+ cells without conditioning and remained on ERT. Afterthe positive results reported by Aiuti et al,12 6 subsequent subjects underwent the procedure under an amended protocol that included withdrawing PEG-ADA and administrating busulfan before infusion of the gene-corrected cells. We demonstrated that only the latter approach led to substantial levels of gene-corrected T-lymphocytes expressing ADA enzyme activity and supporting immune reconstitution.
Methods
Clinical trial protocol
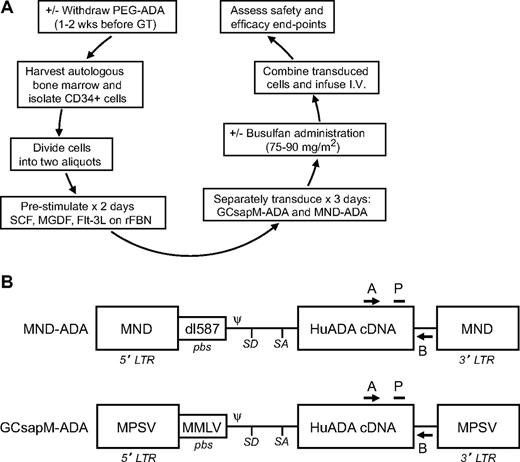
This phase 1/2 clinical trial used retroviral-mediated gene transfer to CD34+ cells from bone marrow of ADA-deficient SCID infants and children (n = 10; Figure 1A). The major eligibility criteria included lack of a suitable matched sibling donor and adequate organ function. The isolated CD34+ cells in each subject were divided into 2 aliquots, each transduced separately with 1 of 2 Moloney murine leukemia virus (MMLV)–based γ-retroviral vectors carrying the human ADA cDNA (MND-ADA and GCsapM-ADA; Figure 1B; supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). After transduction, the 2 cell aliquots were recombined as the final cell product for intravenous infusion. Clinical trial objectives included assessments of safety (toxicities from the procedure and exposure to replication-competent retrovirus [RCR]) and efficacy (transduction/engraftment of HSC, ADA gene expression, and assessment of immune function). Quantitative polymerase chain reaction (qPCR) primers specific for each retroviral vector were used to measure the frequency of cells containing vectors. Subjects were actively followed for the initial 2 years and then were enrolled into a clinical trial to monitor long-term outcome after gene transfer for a 15-year follow-up mandated by the US Food and Drug Administration. The 2 clinical trial performance sites were the Children's Hospital Los Angeles (CHLA) Bone Marrow Transplant Unit and the Clinical Center of the National Institutes of Health (NIH), Bethesda, MD. Cell processing followed a common protocol at both sites.
Experimental schema and maps of retroviral vectors carrying the normal human ADA cDNA. (A) The experimental schema for the clinical trial is shown. Flt-3L indicates Flt-3 ligand; MGDF, megakaryocyte growth and differentiation factor; rFBN, recombinant fibronectin; and SCF, stem cell factor. (B) The elements of the proviral forms of the 2 retroviral vectors used to transfer normal human ADA cDNA are depicted. A indicates common qPCR primer; B, vector-specific qPCR primer; dl587, endogenous murine retrovirus dl587rev; LTR, long terminal repeat; MMLV, Moloney murine leukemia virus; MND, MPSV LTR, ncr-deleted, coupled to dl587rev pbs; MPSV, myeloproliferative sarcoma virus; P, common qPCR probe; pbs, primer-binding site; Ψ, packaging signal; SA, splice acceptor site; and SD, splice donor site.
Experimental schema and maps of retroviral vectors carrying the normal human ADA cDNA. (A) The experimental schema for the clinical trial is shown. Flt-3L indicates Flt-3 ligand; MGDF, megakaryocyte growth and differentiation factor; rFBN, recombinant fibronectin; and SCF, stem cell factor. (B) The elements of the proviral forms of the 2 retroviral vectors used to transfer normal human ADA cDNA are depicted. A indicates common qPCR primer; B, vector-specific qPCR primer; dl587, endogenous murine retrovirus dl587rev; LTR, long terminal repeat; MMLV, Moloney murine leukemia virus; MND, MPSV LTR, ncr-deleted, coupled to dl587rev pbs; MPSV, myeloproliferative sarcoma virus; P, common qPCR probe; pbs, primer-binding site; Ψ, packaging signal; SA, splice acceptor site; and SD, splice donor site.
The protocol (clinicaltrials.gov ID NCT00018018) was approved by the institutional review boards and institutional biosafety committees at CHLA and NIH by the NIH Office of Biotechnology Activities Recombinant DNA Advisory Committee (9908-337). It was conducted under BBIND 8556 from the US Food and Drug Administration. The National Heart, Lung, and Blood Institute Cell Therapy/Gene Therapy Data Safety Monitoring Board served as the safety monitoring entity. All human trial participants (or legal guardians for minors) provided written informed consent in accordance with the Declaration of Helsinki.
Retroviral vectors
Two similar MMLV-based γ-retroviral vectors were used to express the human ADA cDNA in the absence of a selectable marker gene (Figure 1B). In the GCsapM-ADA vector, ADA cDNA expression is driven by the nonmodified MPSV LTR and the MMLV primer-binding site (pbs) is retained.21 In the MND-ADA vector, ADA cDNA expression is driven by the MND LTR, which consists of the myeloproliferative sarcoma virus LTR with a 66-bp negative control region deletion eliminating a YY-1 binding site, and substitution of the MMLV pbs with the pbs from an endogenous murine retrovirus (dl597rev; supplemental Methods).18-20
Clinical monitoring for toxicities
Subjects were monitored for safety and toxicity during and after the administration of busulfan, when given (supplemental Methods) and after the reinfusion of the transduced cells. Clinical complications and abnormal laboratory values were graded with the Division of AIDS (National Institute of Allergy and Infectious Diseases, NIH) Table for Grading Severity of Pediatric Adverse Experiences, April 1994. Subjects were tested for exposure to RCR by qPCR assay for gibbon ape leukemia virus sequences in peripheral blood mononuclear cell (PBMC) samples (baseline and after 3, 6, 12, and 24 months) at the National Gene Vector Laboratory. Tested samples from all 10 subjects were below the limit of detection (< 10 gibbon ape leukemia virus copy number per 0.2 μg of DNA).
Immunologic monitoring and ADA enzyme assays
Immune function tests and ADA assays are described in the supplemental Materials.
qPCR determination of vector copy number
qPCR was performed with primers and probe designed to specifically amplify integrated proviral sequences of either the MND-ADA or the GCsapM-ADA vector. A common sense primer and a common TAMRA probe were used to detect both MND-ADA and GCsapM-ADA; the antisense primers were specific to their respective vectors. All reactions used Universal Master Mix (Applied Biosystems, or ABI), 350 ng of template DNA, and were run under default conditions on the 7900 or 7500 Sequence Detector System (ABI). Results were compared with copy number standards (supplemental Methods).
Data and statistical analyses
The Pearson correlation (r) was used to assess the linear correlation between the administered busulfan dosage (mg/m2) and the resulting busulfan AUC. Continuous outcomes, such as vector copy number (VCN), PBMC ADA activity, erythrocyte %dAXP, absolute lymphocyte counts, and serum IL-7 levels, were collected repeatedly over 2 years. To account for the correlated/unbalanced structure of the data, we performed generalized multivariate linear regression analysis on these outcomes.22 This approach allowed us to model the mean response as a smoothly changing function of time and other experimental factors. Within this framework, we performed estimation and hypothesis testing of the mean responses at different time point and various experimental covariate settings. For all statistical investigations, tests for significance were 2-tailed, with a statistically significant P value threshold of .05. Statistical analyses were performed with SAS Version 9.2.23
Results
Subjects
Ten subjects (ages 15 months to 20 years) with ADA-deficient SCID were enrolled between 2001 and 2009 (Table 1). All initially were diagnosed on the basis of biochemical demonstration of severe ADA deficiency. Mutations in the ADA gene locus were documented in 9 subjects, and most are known severe null or minimal activity mutations,24 with 1 patient not genotyped. Patients had been treated with ERT since diagnosis until enrollment in the study, from 1-14 years. In the first 4 subjects, enrolled in 2001-2002 (subjects 201-204), ERT was not discontinued and busulfan was not administered. In the last 6 subjects, enrolled between 2005 and 2009 (subjects 301-306), ERT was withdrawn and busulfan was administered before reinfusion of gene-modified CD34+ cells.
CD34+ cell dosages and transduction efficiency
The numbers of cells transduced and reinfused were limited by the amount of BM harvested (10-15 mL/kg), and the resultant numbers of CD34+ cells collected, isolated, and present at the end of transduction. The CD34+ cell dosages ranged from 0.7 to 9.8 × 106 cells/kg (Table 1). There were no toxicities associated with the reinfusion of the cells in any of the subjects.
The VCNs of GCsapM-ADA and MND-ADA detected by qPCR in each subject's transduced cells before recombining for reinfusion were between 0.1 and 1.8 VCNs/cell (Table 1). The transduced CD34+ cells had ADA enzyme activity in the range present in normal human PBMCs (58-128nM/min/108 cells), demonstrating expression by the vector (Table 1). There was not a consistent advantage to transduction by either vector as assessed by these in vitro assays.
Busulfan administration
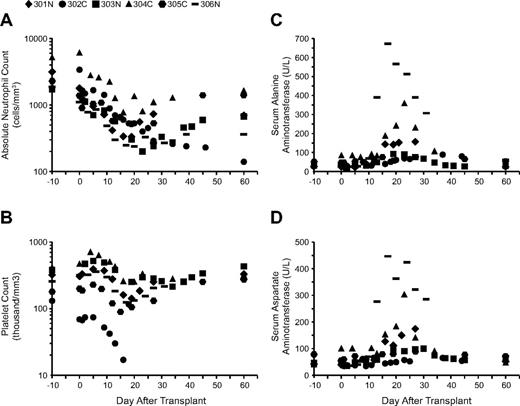
Six subjects were given busulfan (65-90 mg/m2) after bone marrow harvest and before infusion of gene-modified cells to enhance engraftment. Busulfan levels were measured and used to calculate the area-under-the-curve (AUC) for busulfan exposure. We observed highly consistent pharmacokinetics with a strong correlation between the administered dose (mg/m2) and the resulting busulfan AUC achieved (r = 0.95, P = .003; supplemental Table 1). In the first 3 subjects (301-303), a busulfan dosage of 75 mg/m2 was given (equating to 2.8-3.1 mg/kg), with AUC of 3006-3034μM*min. Subject 304C was obese at > 20% above the ideal body weight (IBW) for height and had a history of hepatosteatosis with mildly increased levels of serum transaminases at baseline. Therefore, the dosage of busulfan was adjusted on the basis of IBW to 65 mg/m2 (or 1.9 mg/kg). The resulting busulfan AUC (2377μM*min) was lower for this subject than for the 3 recipients of 75 mg/m2, suggesting that the IBW dosage adjustment may not have been needed. Nonetheless, this subject had relatively high elevations of her serum transaminases at 1 month (Figure 2), which may have been more severe had she received a nonadjusted dose. In an effort to increase engraftment of gene-corrected cells in the last 2 subjects (305C, 306N), the busulfan dosages were increased to 90 mg/m2 (∼ 4.0 mg/kg), which produced concordantly greater AUC (4060-4873μM*min).
Hematologic values and serum transaminase levels after busulfan administration. (A) ANC, (B) platelet counts, (C) serum alanine aminotransferase, and (D) serum aspartate aminotransferase levels over 2 months.
Hematologic values and serum transaminase levels after busulfan administration. (A) ANC, (B) platelet counts, (C) serum alanine aminotransferase, and (D) serum aspartate aminotransferase levels over 2 months.
No clinical toxicities were observed as a result of the busulfan; specifically, there was no associated nausea, vomiting, anorexia, seizures, or hair loss. All of the subjects who received busulfan experienced transient neutropenia, thrombocytopenia, and elevation of liver enzymes. The nadir of neutropenia (between 200 and 700/mm3) occurred between days 22 and 26 after busulfan dosing (Figure 2A), except for subject 302C, who is discussed in “Clinical outcomes.” No adverse consequences of neutropenia were observed. However, brief courses of G-CSF treatment were used in subjects 301N, 303N, and 306N to help resolve low absolute neutrophil counts (ANCs) that persisted 70-100 days after the administration of busulfan. The nadirs of thrombocytopenia were more variable and occurred between days 18 and 37 after dosing, with the lowest recorded platelet count of 97 000/mm3 in 305C, who had the greatest busulfan AUC (Figure 2B). Subject 304C, who received the lowest dose of busulfan (65 mg/m2) and had the lowest busulfan AUC, experienced the smallest decreases in neutrophil and platelet counts. Peak elevation of the liver enzymes alanine aminotransferase and aspartate aminotransferase occurred between days 20 and 33 after busulfan administration (Figure 2C-D). In subjects 304C and 306N, the increase in liver enzymes reached grade 3 on the DAIDS toxicity scale; however, liver synthetic function remained consistently unaffected, and the transaminitis resolved without intervention by 2 months after treatment.
Clinical outcomes
The 4 subjects who remained on PEG-ADA and did not receive busulfan (201-204) experienced no adverse events. They remain clinically stable on continued ERT, now 9-10 years after the procedure. In contrast, the 6 subjects who were withdrawn from ERT and received busulfan (301-306) experienced transient neutropenia and thrombocytopenia and moderate but self-limited increases in serum transaminases, as described previously. Other complications occurred in 3 subjects, as described in the paragraphs to follow.
Three of the subjects (301N, 303N, and 305C) remain clinically well and have been off ERT since the procedure, now 3-5 years later. These subjects remain on trimethoprim/sulfamethoxazole prophylaxis for Pneumocystis jiroveci and intravenous γ-globulin (IVIg), except for subject 301N, who has been off IVIg since 6 months after treatment.
Three of the subjects experienced adverse events after the procedure. Subject 302C, who has been reported previously,25 was found to have trisomy 8 mosaicism in bone marrow cells (including a sample taken before treatment with busulfan) and underwent a MUD HSC transplantation 8 months after the gene transfer procedure and is clinically well. Data from this subject are not included in the subsequent analyses.
Two subjects developed infectious complications 4-5 months after the procedure. 304C was noted to have fever, otitis media, and left lower-lobe pneumonia. She was hospitalized for intravenous antibiotics, which resulted in resolution of the symptoms without sequelae. 306N developed fever and diarrhea on day 135 after the procedure and was admitted to the hospital. Adenovirus was detected by PCR in stool and blood samples and became undetectable after the patient received 11 weeks of intravenous cidofovir. As per protocol, both 304C and 306N were restarted on ERT at the time of the infectious complications to hasten immune reconstitution, and ERT has continued through the 24 months of follow-up.
Quantification of VCN in PBMC and granulocytes
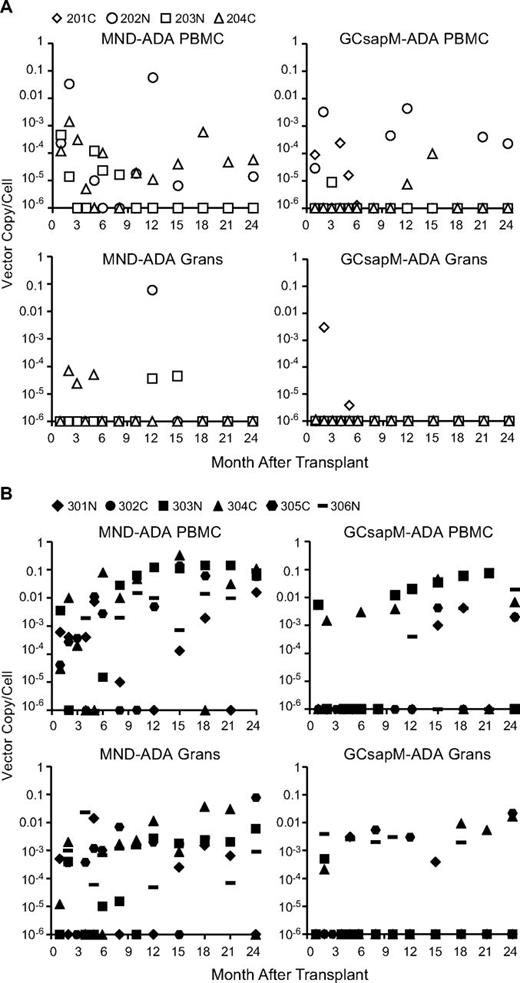
To assess the transduction and engraftment of ADA gene-modified CD34+ cells, blood samples were obtained at serial time points, and VCNs in PBMC and granulocytes were determined by the use of qPCR. In the 4 subjects who did not receive busulfan conditioning (201-204), there was only minimal and short-term presence of vector-containing granulocytes, with no vector in the myeloid cells beyond one year (Figure 3A). For the 2 older subjects, treated at 15 and 20 years of age (201C and 203N), there was no detectable vector-marking in PBMC beyond the first year. In contrast, the 2 younger subjects, treated at 6 and 4 years of age (202N and 204C), did have persistence of PBMC containing both the MND-ADA and GCsapM-ADA vectors, albeit at very low frequencies (10−3-10−5 VCN/cell). Vector-marked PBMC have remained intermittently detectable at these levels for 8-9 years of evaluation (data not shown).
qPCR measurements of the average vector copy/cell in blood cell samples obtained after transplantation. Separate qPCR analyses were performed on granulocytes (Grans) and PBMC fractions with primer/probe sets specific for the MND-ADA vector or the GCsapM-ADA vector provirus. (A) Results from subjects not receiving busulfan conditioning and remaining on ERT (201-204) and (B) results from subjects having ERT withdrawn and receiving busulfan before transplantation (301-306).
qPCR measurements of the average vector copy/cell in blood cell samples obtained after transplantation. Separate qPCR analyses were performed on granulocytes (Grans) and PBMC fractions with primer/probe sets specific for the MND-ADA vector or the GCsapM-ADA vector provirus. (A) Results from subjects not receiving busulfan conditioning and remaining on ERT (201-204) and (B) results from subjects having ERT withdrawn and receiving busulfan before transplantation (301-306).
The subjects who received busulfan (301-306) showed significantly greater levels of vector-containing cells in both the granulocyte and PBMC fractions (P < .05 from month +10 onward, Figure 3B). In this group, the levels of vector marking in PBMC were 100-fold greater (0.01-0.1 VCN/cell, or 1%-10% of cells) than in granulocytes (0.001 VCN/cell, or 0.1% of cells; P = .0017). Interestingly, the frequencies of vector-marked cells in the 2 subjects who had ERT resumed (304C and 306N) were similar to those for whom ERT was not restarted (301N, 303N, 305C, P = .996).
There was a general trend for greater levels of PBMC and granulocytes marked with the MND-ADA vector than with the GCsapM-ADA vector (P = .0111); only one subject showed persistent gene-marking in granulocytes with GCsapM-ADA beyond 1 year, whereas all subjects had persistence of granulocytes marked with MND-ADA (Figure 3B). Analysis of the relative gene marking by the 2 vectors in the individual subjects showed a modestly greater level with MND-ADA than with GCsap-M-ADA (supplemental Figure 1). T-cell clones grown from peripheral blood of 3 subjects (301N, 303N, 305C) were found to harbor the MND-ADA vector with greater frequency than the GCsapM-ADA vector (119 vs 11; supplemental Table 2 and supplemental Figure 2). No significant difference was noted, however, in the ADA enzymatic activity provided by the 2 vectors in these isolated clones.
DNA from PBMC samples that had vector marking levels > 1% was examined by linear amplification–mediated (LAM)–PCR (National Gene Vector Biorepository) to amplify junctions of vector integrants and flanking chromosomal sequences. No progressive clonal expansions were seen by agarose gel electrophoresis of LAM-PCR products (data not shown). We also used nonrestrictive LAM-PCR and high-throughput sequencing to determine the patterns of vector integration sites in PBMC from the conditioned subjects (supplemental Methods). We found relatively low diversity of integration sites in PBMCs from each subject, ranging from 22 to 145 unique integrants per subject, more than 2 years after transplantation (supplemental Figure 3). Stable clones with vectors near known common integration sites (eg, EVI-1, LMO2) were observed, without clinical consequences, during the 2-year observation period.
PBMC ADA enzyme activity and RBC dAXP levels
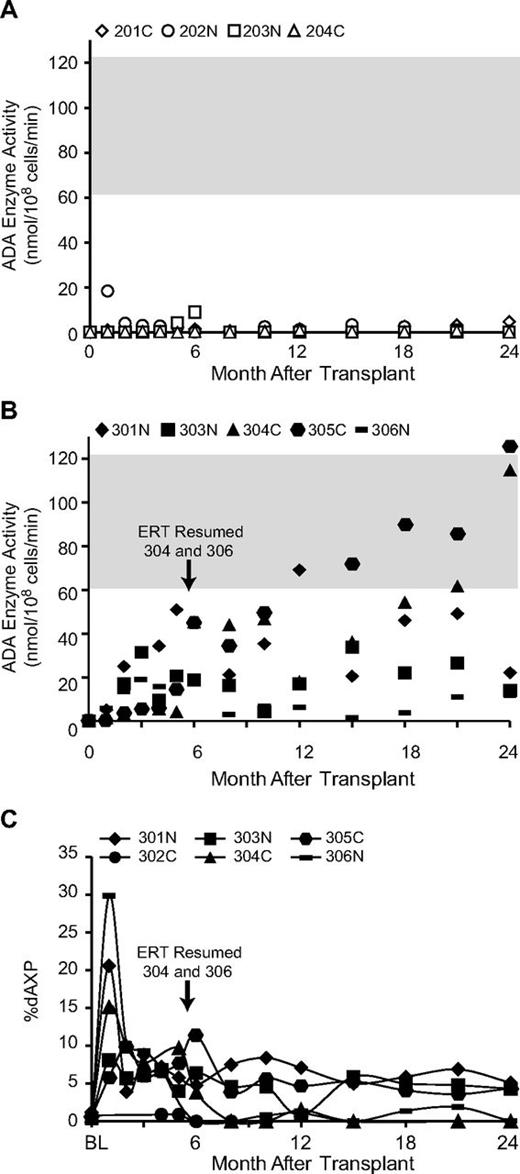
Before treatment, PBMCs had essentially no detectable ADA activity, consistent with the severe deficiency in these SCID subjects. In the 4 subjects who did not receive busulfan and remained on ERT (201-204), there were minimal increases in the PBMC ADA activity in the months after transplantation (Figure 4A). In contrast, PBMC ADA activity of each of the subjects who received busulfan and had ERT withdrawn (301-306) showed a gradual increase in the months after transplantation to levels from half-normal to normal range (Figure 4B). The levels of ADA activity in PBMCs were significantly greater from month +5 onward in recipients of busulfan than in subjects not receiving busulfan (P < .05). Notably, subject 304C, who went back on ERT after 5 months, had a similar increase in PBMC ADA activity, suggesting the absence of negative effects of ERT on the persistence and expansion of engrafted gene-corrected cells. In contrast, ADA 306N who also went back on ERT showed substantially less increase in PBMC ADA activity (∼ 12 units at 24 months).
ADA enzymatic activity in PBMC and % deoxyadenine nucleotides in erythrocytes. ADA enzyme activity in PBMC was measured biochemically. The graphs show (A) the values from subjects not receiving busulfan conditioning and remaining on ERT (201-204) and (B) the values from subjects having ERT withdrawn and receiving busulfan before transplantation (301-306). The normal reference range for the ADA enzyme assay in human PBMC is indicated (gray-shaded horizontal bar). (C) Adenine and deoxyadenine metabolites were measured in erythrocytes by high-pressure liquid chromatography and the percentage that were deoxyadenosine nucleotides (dAMP + dADP + dATP) were plotted as %dAXP for the subjects in the group receiving busulfan (301-306). The times when ERT was resumed for subjects 304 and 306 are indicated.
ADA enzymatic activity in PBMC and % deoxyadenine nucleotides in erythrocytes. ADA enzyme activity in PBMC was measured biochemically. The graphs show (A) the values from subjects not receiving busulfan conditioning and remaining on ERT (201-204) and (B) the values from subjects having ERT withdrawn and receiving busulfan before transplantation (301-306). The normal reference range for the ADA enzyme assay in human PBMC is indicated (gray-shaded horizontal bar). (C) Adenine and deoxyadenine metabolites were measured in erythrocytes by high-pressure liquid chromatography and the percentage that were deoxyadenosine nucleotides (dAMP + dADP + dATP) were plotted as %dAXP for the subjects in the group receiving busulfan (301-306). The times when ERT was resumed for subjects 304 and 306 are indicated.
ADA-deficient SCID patients accumulate deoxyadenine nucleotides (dAXP) in erythrocytes, and the percentage of adenine nucleotides that are deoxyadenine nucleotides (%dAXP) are measured routinely to follow the efficacy of ERT. For the subjects remaining on ERT continuously (201-204), the erythrocyte %dAXP remained at low levels (< 1%), indicating adequate detoxification (data not shown). In contrast, the levels of dAXP increased sharply on ERT withdrawal in subjects 301-306 (Figure 4C). The %dAXP values for the 3 subjects who remained off ERT for the 2 years after transplantation (301N, 303N, 305C) remained in the range of ∼ 5%, which is similar to levels seen in ADA-deficient SCID patients who have undergone successful allogeneic HSC transplantation.26 The 2 subjects who resumed ERT (304C and 306N) had significantly lower levels of RBC dAXP (P < .05 from month +18 onward).
Immunologic outcomes
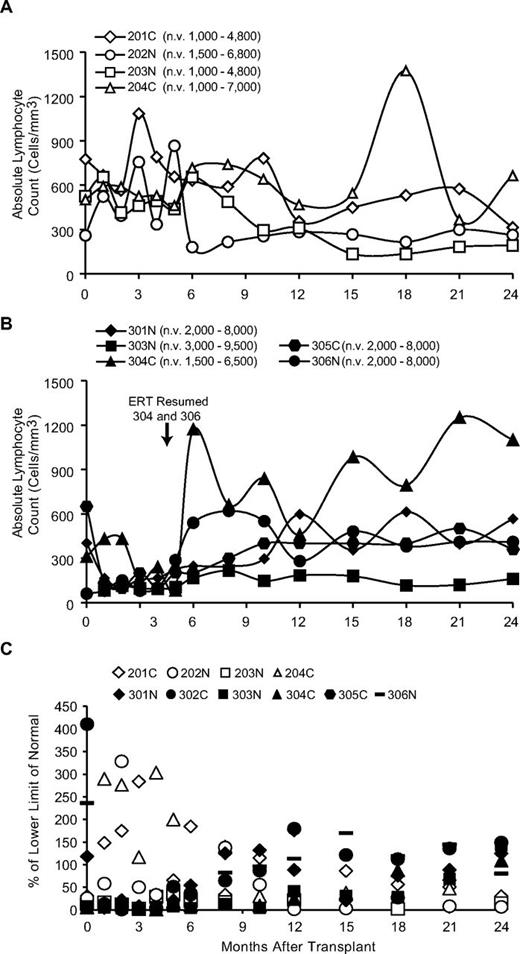
The nonconditioned subjects remaining on ERT had stable ALC in the subnormal range of ∼ 300-500/mm3, which is typical for patients on long-term ERT (Figure 5A).7 In contrast, in the conditioned subjects for whom ERT was stopped, the ALC decrease sharply in the first months, likely because of combined effects of busulfan administration and toxicity from deoxynucleotide accumulation on ERT withdrawal. In the 3 subjects who remained off ERT (301N, 303N, and 305C), the ALC increased over 6-24 months (∼ 300-1000/mm3). The 2 subjects who had their ERT resumed at 5 months (304C and 306N) showed more rapid increases in ALC, which stabilized at levels similar to those before ERT was interrupted (Figure 5B). The ALC for the group that had ERT stopped were significantly lower than in those remaining on ERT over the first 10 months (P < .05).
Absolute lymphocyte counts and proliferative responses after gene transfer. (A) Results from subjects not receiving busulfan conditioning and remaining on ERT (201-204) and (B) results from subjects having PEG-ADA ERT withdrawn and receiving busulfan before transplantation (301-306). n.v. indicates normal values for age range. The time when ERT was resumed for subjects 304 and 306 is indicated. (C) Proliferative responses to PHA of PBMC from subjects over time. Open symbols represent subjects remaining on ERT and not receiving busulfan; filled symbols represent subjects receiving busulfan with ERT stopped.
Absolute lymphocyte counts and proliferative responses after gene transfer. (A) Results from subjects not receiving busulfan conditioning and remaining on ERT (201-204) and (B) results from subjects having PEG-ADA ERT withdrawn and receiving busulfan before transplantation (301-306). n.v. indicates normal values for age range. The time when ERT was resumed for subjects 304 and 306 is indicated. (C) Proliferative responses to PHA of PBMC from subjects over time. Open symbols represent subjects remaining on ERT and not receiving busulfan; filled symbols represent subjects receiving busulfan with ERT stopped.
Lymphocyte subset values at 24 months after the procedure are listed in Table 2 (see also supplemental Figure 4 for serial values). The absolute numbers of CD3+ T cells and T-cell subtypes (CD4+, CD8+, %CD45RA+/CD4+, ie, “naive”) were below normal in all subjects, whether receiving ERT continuously or having had ERT withdrawn before gene transfer. T-lymphocyte subsets were in the ranges of CD3+, 100-500/mm3; CD4+, 75-200/mm3; and CD8+, 100-400 cells/mm3. Interestingly, subject 304C who restarted ERT within 5 months of the gene transfer had the greatest numbers of T cells, B cells, and natural killer (NK) cells, with normal IgA, suggesting the dual treatments had additive effects. However, 306N was also restarted on ADA ERT at 5 months yet had lower lymphocyte numbers.
In vitro lymphocyte proliferation responses to phytohemagglutinin (PHA) remained defective in the first cohort of subjects (201-204), who did not have significant numbers of gene-corrected cells (Table 2). The PHA responses were significantly greater at 24 months in the second cohort (301-306; P = .024), with a general trend to greater PHA responses at times after 1 year (Figure 6C). We also assessed the diversity of T-cell receptor rearrangement by spectratype in some subjects and observed broad, nonrestricted repertoires (supplemental Figure 5). Analysis of T-cell receptor excision circles did not show an advantage for either treatment on this measure of thymic reconstitution (Table 2). There was a strong association with achieving greater levels of PBMC-expressing ADA and the presence of CD45RA/CD4+ naive T cells (supplemental Figure 4).
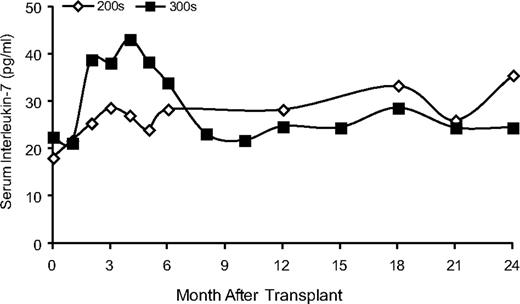
Serum IL-7 levels. Stored serum samples were used to measure levels of IL-7 by ELISA. The average IL-7 levels for the subjects remaining on continuous ERT (201-204) and for the subjects withdrawn for ERT and receiving busulfan (300 series) are shown.
Serum IL-7 levels. Stored serum samples were used to measure levels of IL-7 by ELISA. The average IL-7 levels for the subjects remaining on continuous ERT (201-204) and for the subjects withdrawn for ERT and receiving busulfan (300 series) are shown.
The absolute numbers of B cells were subnormal in all of the subjects (Table 2). Two of the subjects were not receiving IVIg replacement and had IgG levels in the normal range. One of the 6 subjects on ERT (203N) was off IVIg. Of the 3 evaluable conditioned subjects, restoration of serum Ig production was complete in only 1 subject (301N), who also responded successfully to immunizations against clinical vaccines to tetanus, diphtheria, Haemophilus influenzae, and poliovirus antigens. 303N had low levels of IgA and IgM, whereas 305C had a normal level of IgA but low IgM. Of the 2 subjects who had resumed ERT at 5 months, one had normal levels of IgA and IgM (304C), and one continued to have low levels of Ig (306N). NK-cell numbers were in the normal range in only 1 of the 4 nonconditioned subjects (202N) and in 1 of the 2 who resumed ERT (304C), and were below normal in the 3 who remained off ERT (301N, 303N, and 305C).
Serum IL-7 levels
Increased serum IL-7 levels have been reported in lymphopenic patients, either untreated SCID patients or after HSC transplantation.30 We measured serum IL-7 in subject samples. Subjects 201-204 maintained relatively constant IL-7 levels in the months after their procedures (Figure 6). In contrast, subjects who were withdrawn from ERT (301-306) showed transient 2-fold increases in serum IL-7 levels in the first 2-6 months after gene transfer. After reaching maximum values at +4 months, the IL-7 serum concentration declined to levels similar to those measured in the subjects who had remained on ERT. The changes in serum IL-7 levels in the subjects withdrawn from ADA ERT varied reciprocally with the absolute lymphocyte counts as they decreased after PEG-ADA was withdrawn and then increased during the next 6-9 months. These results are consistent with models in which IL-7 levels are modulated by the rate of consumption by lymphocytes and correlate inversely with ALC.31
Discussion
This trial performed a direct comparison between 2 different approaches to gene therapy for ADA-deficient SCID: the first cohort of 4 subjects did not receive cytoreductive conditioning before reinfusion of transduced bone marrow CD34+ cells and continued to receive ERT, whereas the second cohort of 6 subjects received nonmyeloablative conditioning with busulfan and had ERT withdrawn. Other relevant factors, such as the γ-retroviral vectors used and the method for in vitro gene transduction of the bone marrow cells, remained constant between the 2 cohorts. Clearly, only the protocol with administration of busulfan and withdrawal of PEG-ADA led to efficacy, as shown by the long-term presence of gene-containing blood cells expressing ADA enzyme activity, which afforded partial immune reconstitution in the absence of exogenous ADA ERT. The absence of conditioning and continuation of ERT did not lead to any adverse effects, but essentially no efficacy was observed. These findings are in accord with those results from previous trials in Italy11,12 and the United Kingdom14,16 in which nonmyeloablative conditioning and ADA ERT withdrawal were performed and subjects realized immune reconstitution. Neither of these previous trials contained a direct comparative group that did not, so the benefits of the approach were determined by comparison with earlier studies performed under markedly different conditions.
The relative contributions of the 2 changed variables (cytoreductive conditioning and ERT withdrawal) are not easily discerned. The administration of busulfan almost certainly was responsible for the greater levels of gene-containing blood cells achieved, by allowing engraftment and persistence of more of the transduced CD34+ cells. The withdrawal of PEG-ADA may have enhanced the relative frequencies of gene-corrected T lymphocytes by leading to lowered numbers of noncorrected lymphocytes, which would dilute the gene-containing cells measured by qPCR. The subjects in the second cohort who had ERT withdrawn developed severe lymphopenia in the first months after transplantation that was associated with increased serum IL-7 levels, and this may have driven the expansion of gene-corrected lymphocytes. Subjects remaining on ERT did not become lymphopenic, had stable levels of IL-7, and did not show increases in lymphocyte numbers.
Interestingly, 1 of 2 subjects who had ERT withdrawn with gene transfer but then restarted 6 months after the procedure (304C) developed similar levels of gene-marked cells as subjects who remained off PEG-ADA (301N, 303N, 305C). This observation implies that PEG-ADA does not significantly blunt the production of gene-containing lymphocytes or their absolute number but merely lowers measurements of their relative frequency by diluting them with noncorrected lymphocytes supported in trans by ERT. On the basis of the absence of a deleterious impact of ERT on production of gene-corrected lymphocytes, it may be advisable to continue ERT for some months after gene transfer to avoid lymphopenia and the associated risks of infection. Of note, 2 children with ADA-deficient SCID were treated in Japan in 2003-2004 by γ-retroviral–mediated gene transfer to bone marrow CD34+ cells after ERT withdrawal but without pretransplantation chemotherapy.32 These patients slowly developed gene-corrected T cells, although full immune recovery was not achieved during a 6-year follow-up period, leading the investigators to suggest that both conditioning and the absence of ERT play roles in the kinetics and extent of immune reconstitution.
The partial cytoablative procedure with 3-4 mg/kg busulfan was well tolerated clinically and was minimally toxic. In 4 of 6 subjects treated with busulfan, we observed prolonged neutropenia that promptly responded to G-CSF if given. The persistence of low ANC (200-500/mm3) at 70-100 days after transplantation in the 3 subjects who eventually recovered neutrophil counts in response to G-CSF may be explained as a consequence of the myeloid dysplasia features that have been recognized recently to accompany ADA deficiency.33
We also observed transitory elevation of transaminases that reached values over 5 times normal in 2 subjects (304C and 306N), who had previous histories of mild hepatosteatosis or hepatomegaly of undetermined nature. Although these complications were self-limited and without clinical sequelae, they are significant in that they did not correlate with AUC of busulfan and may reflect the specific hepatic sensitivity of some ADA-deficient patients.34 Besides these complications, our observations confirm that the dosage of busulfan first used by investigators in Milan affords an effective enhancement of engraftment, without significant acute toxicity. An interim analysis of data obtained in subjects 301-304 indicated that administration of 75 mg/m2 busulfan resulted in 2-3 mg/kg dose and modest engraftment of gene-marked cells. For this reason, we increased the dosage to 90 mg/m2 for the following subjects. This resulted in 4-5 mg/kg dosage (supplemental Table 1), without increased toxicity.
This trial also involved a direct comparison of 2 retroviral vectors differing mainly by the presence or absence of the wild-type MMLV pbs, a sequence associated with profound silencing in murine HSC and embryonic stem cells.35-37 Although we did not have direct evidence for silencing of either vector, the frequency of PBMC and granulocytes containing the MND-ADA vector generally was greater than that of cells carrying the GCsapM-ADA vector, despite similar initial transfer by the 2 vectors in the CD34+ cells. In addition, the majority of T-cell clones isolated from treated subjects contained the MND-ADA vector. These findings suggest that the alternative pbs carried by the MND-ADA vector conveyed favorable expression features that allowed it to outperform the GCsapM-ADA vector in production or survival of ADA gene-corrected immune cells. On the basis of these observations, we are now performing a phase 2 study for ADA-deficient SCID using only the MND-ADA vector (NCT00794508), but retaining the key features of the phase 1 trial with low dose busulfan and PEG-ADA withdrawal before treatment.
The outcomes for the 6 subjects treated with cytoreductive conditioning in the absence of ERT are encouraging: after 3-5 years, 3 remain well without ERT. From this group, as well as 8 additional subjects treated under the ongoing phase 2 trial, we have observed that younger subjects (< 2-4 years) yield greater numbers of bone marrow CD34+ cells allowing greater numbers of transduced cells to be transplanted and achieve greater numbers of T, B, and NK cells expressing ADA enzyme, compared with the older subjects. This observation also may explain the differences in outcome between the subjects reported here and those reported in the first trial from Milan, where 8 of 10 subjects achieved significant immune reconstitution.11 Indeed, for the cohort treated in Milan, the average age of the treated patients was lower and the average CD34+ cell dose was greater than for our subjects.
Importantly, none of the subjects in any of the clinical trials of gene therapy for ADA-deficient SCID that used this type of approach (n = 38) have encountered the complications from insertional oncogenesis that occurred in trials for other disorders.38-40 It is not known whether the better safety profile in the ADA-deficient SCID trials is merely fortuitous or reflects real biologic differences. The vector integration site analyses we performed revealed oligoclonal reconstitution, suggesting that a relatively low number of transduced HSC engrafted; this may underlie the incomplete immunologic benefits achieved. Further follow-up of these and additional subjects will be needed to understand long-term benefits and risks of autologous transplantation of gene-modified HSC compared with alternatives of allogeneic HSCT or long-term ERT.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kathy Wilson, MSN, Renna Killen, MSN, Mirna Sweeney, RN, the CHLA Clinical Immunology Laboratory staff, and the nurses of the BMT Unit at CHLA for their important roles in patient care. At the National Institutes of Health, invaluable contributions were made by Dr Cynthia Dunbar, the Cell Processing Section of the Department of Transfusion Medicine, the nurses in 1NW of the Clinical Center, and Julia Fekecs for providing the medical artwork. Clinical-grade GCsapM-ADA and MND-ADA supernatants used in the trial were produced by W. Jay Ramsey, Laura Tuschong, and Linda Muul in the Clinical Gene Therapy Branch of the National Human Genome Research Institute, National Institutes of Health. Estuardo Aguilar Cordova and Ken Cornetta provided support with production of clinical-grade vector supernatants. Takara Shuzo Inc, Amgen Inc, and Immunex, all provided essential reagents for CD34+ cell transduction. The National Gene Vector Laboratories and the National Gene Vector Biorepository provided clinical trial support with assays for RCR and LAM-PCR. The UCLA Eli & Edythe Broad Center of Regenerative Medicine & Stem Cell Research Flow Cytometry Core and High Through-Put Sequencing Core were essential for these studies.
This study was supported by the following awards: a Clinical Research Award from the Saban Research Institute of Children's Hospital Los Angeles, a Distinguished Clinical Scientist Award (2000-654) from the Doris Duke Charitable Foundation, an NHLBI SCOR grant to R. Parkman (1P50 HL54850), and FDA 1 RO1 FD003005. The Clinical Gene Therapy Core Laboratory of the CHLA/USC General Clinical Research Center (MO1 RR0043) was vital to performance of this trial and the UCLA GCRC also helped support this study (MO1 RR000865). Sigma-Tau Pharmaceuticals provides research grant support to M.S.H. This study was also supported in part by intramural funds of NHGRI, NIDDK, and NCI.
National Institutes of Health
Authorship
Contribution: F.C. and D.B.K. developed the clinical trial, codirected the studies, participated in subject enrollment, treatment, and evaluations, and primarily wrote the paper; K.L.S., E.G., G.J.J., B.C.E., and G.M.P. served as clinical and regulatory coordinators for the study; R.S., S.H.S., and C.K. participated in subject enrollment, treatment, and evaluations; J.F.T., K.I.W., G.M.C., N.K., A.S., H.A. A., A.S.W., H.M.R., C.M.D., C.H., R.G.R. provided clinical care to the subjects and participated as clinical coinvestigators; L.M., D.C., C.C., X.-J.Y., P.-Y.F., E.G., A.C., M.S., O.O.Y., A.B., G.B., and J.A.I. performed clinical HSC processing and/or laboratory end point studies; X.W. and D.G performed statistical analyses; and M.S.H., R.M.B., and R.P. participated in clinical trial development and analysis.
Conflict-of-interest disclosure: M.S.H. receives grant support from Sigma-Tau Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Donald B. Kohn, MD, 3163 Terasaki Life Science Bldg, 610 Charles E. Young Dr E, Los Angeles, CA 90095; e-mail: dkohn@mednet.ucla.edu.