Abstract
Sickle cell pain includes 3 types: acute recurrent painful crises, chronic pain syndromes, and neuropathic pain. The acute painful crisis is the hallmark of the disease and the most common cause of hospitalization and treatment in the emergency department. It evolves through 4 phases: prodromal, initial, established, and resolving. Each acute painful episode is associated with inflammation that worsens with recurrent episodes, often culminating in serious complications and organ damage, such as acute chest syndrome, multiorgan failure, and sudden death. Three pathophysiologic events operate in unison during the prodromal phase of the crisis: vaso-occlusion, inflammation, and nociception. Aborting the acute painful episode at the prodromal phase could potentially prevent or minimize tissue damage. Our hypothesis is that managing these events with hydration, anti-inflammatory drugs, aggressive analgesia, and possibly vasodilators could abort the crisis and prevent or minimize further damage. Chronic pain syndromes are associated with or accompany avascular necrosis and leg ulcers. Neuropathic pain is not well studied in patients with sickle cell disease but has been modeled in the transgenic sickle mouse. Management of sickle cell pain should be based on its own pathophysiologic mechanisms rather than borrowing guidelines from other nonsickle pain syndromes.
Introduction
Sickle cell disease (SCD) has been primarily considered a disease of children with a few patients surviving to adulthood. Between 1910 and 1950, the median survival of patients with SCD was < 20 years of age.1 Sudden death occurred in ∼ 41% of the patients, whereas 29% deaths occurred within 24 hours after the onset of painful crises.2 By 1980, 50% of children survived to 20 years of age; and by 2009, survival to age 20 years increased to 85% of children.2
However, if we rewind the clock by more than a century when SCD was first described in the United States, we find that the first 4 SCD patients reported were adults with leg ulcers.3-6 Before 1910, there were observations among adult African slaves suggestive of SCD with immunity to malaria and high prevalence of leg ulcers.7 Moreover, autopsy on a slave with history of fever and respiratory illness in 1846 showed absence of the spleen.8 The inevitable question then is: where were the children with SCD in the United States before 1910? There must have been children with SCD. The patient reported by Cook and Meyer5 gave family history of brothers and sisters who died in early life of a disease associated with grave anemia. These children must have lived, suffered, and died prematurely, the dark side of the discovery of sickle cell anemia in the United States.9 It was not until the early 1930s that children with SCD were recognized.
Sickle cell pain between 1930 and 1960
Between 1930 and 1960, the emphasis was on studying the basic science of SCD. This period witnessed quantum leaps in defining the specific molecular signature of SCD with the advent of hemoglobin (Hb) electrophoresis.10-12 Diggs and Chang suggested that the painful sickle cell crises are the result of blockage of the small blood vessels by the abnormal RBCs.13 The major therapeutic goal was to identify methods to prevent the blockage of small blood vessels. Diggs realized that pain was a major problem among patients with sickle cell anemia and used papaverine and acetaminophen to treat it.14 A number of futile therapeutics were introduced, including liver extract, whole liver diet, spleen diet, iron, arsenic, calcium, iodides, alkalis, blood transfusion, urea, cyanate, and hyperbaric oxygen.15-19
In the 1960s, adult patients with severe pain not responsive to oral analgesics were treated in the emergency department (ED). At that time, the only parenteral opioids approved by the US Food and Drug Administration (FDA) for pain management were meperidine and fentanyl citrate. The latter, however, was approved for surgical use only, and meperidine became the drug of choice to treat severe sickle cell pain. Follow-up studies of the Multicenter Study of Hydroxyurea (MSH) in sickle cell anemia showed that meperidine was most often used for painful crises in the ED and hospital and short-acting oxycodone with acetaminophen most often used for pain at home.20,21
Sickle cell pain and opioids: is morphine the ideal and only opioid for sickle cell pain?
A shift in the management of sickle cell pain from meperidine to morphine occurred in the 1990s. Morphine sulfate was approved by the FDA in 1984 and quickly became widely used in the management of cancer pain. A publication by Brookoff and Palomino initiated a trend to treat sickle cell pain by borrowing methods from other disciplines rather than establish treatment based on the specific pathophysiology of the disease itself.22 Brookoff and Palomino reported that using intravenous and controlled release oral morphine instead of meperidine reduced the frequency of hospital admissions of patients with painful crises and their length of stay.22 However, the decrease was probably the result of the patients self-transferring care to other hospitals after the institution of the morphine-only policies in their original hospital!23 This policy conveyed the message to providers that morphine is the only opioid to be used to treat acute painful crises. The meperidine phobia emerged because nor-meperidine, the major metabolite of meperidine, is neurotoxic and may lower the seizure threshold especially in patients with impaired renal function. There are no controlled trials to compare meperidine with morphine in patients with SCD. The incidence of seizures related to the use of meperidine in patients with SCD varies between 1% and 12%.24-26
Although there are no controlled trials to compare the safety and efficacy of different opioids in the management of acute sickle cell crises, patient safety can be maximized by obtaining a detailed history; understanding opioid pharmacology, mechanism of action and side effects; carefully monitoring patients; and individualizing care. Meperidine should not be used to treat acute sickle cell pain in patients with impaired renal function, history of seizure disorder, or those on serotoninergic medications.
Ironically, it turned out that morphine is not a panacea in terms of its own side effects. These include nausea, vomiting, pruritus, constipation, mental changes, tolerance, dependence, hyperalgesia, and addiction. Morphine also causes seizures with a reported prevalence of 1.2% if given in sufficiently large doses.27 Morphine is the most histaminergic opioid28 and is associated with severe pruritus in some patients. Moreover, there have been reports of death with the use of morphine in patients with SCD.29,30 The use of morphine in patients with SCD seems to be associated with acute chest syndrome.31-33 In addition, morphine and morphine-6-glucoronide, a major metabolite of morphine, are both excreted in the urine, and care has to be taken if given to patients with renal failure.
Morphine stimulates diverse neural and non-neural molecular targets (see supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Morphine induces expression of platelet-derived growth factor-BB (PDGF-BB) in human brain and umbilical vein endothelial cells and PDGF receptor-β (PDGFR-β) expression in pericytes.34,35 Morphine also activates vascular endothelial growth factor receptor-2 (VEGFR2), PDGFR-β, sphingosine 1 phosphate receptor 3 (S1P3R), mitogen-activated protein kinase/extracellular signal related kinase (MAPK/ERK)– and cycloxygenase-2 (COX-2) in endothelial cells and the CNS.35-39 Together, these growth factor-like activities of morphine stimulate vascular permeability, endothelial activation, vascular smooth muscle cell pathology, analgesic tolerance, and kidney disease. Studies in the transgenic sickle mouse showed that morphine amplifies renal pathology, stimulates albuminuria, and impairs renal function.36,40-42 These morphine-induced signaling mechanisms suggest that pulmonary edema may perhaps be the result of increased vascular permeability in patients receiving morphine and hydrated or overhydrated at the same time. Alternatively, it could be the result of undiagnosed pulmonary hypertension that can lead to pulmonary edema with aggressive hydration. We speculate that increased vascular permeability induced by morphine may be associated with hemorrhagic stroke in SCD. Considering the endothelial and neural-specific effects, morphine use needs to be critically evaluated for their contribution to organ disease.
Presently, several opioids are available for parenteral use as needed, including morphine, codeine, meperidine, hydromorphone, oxymorphone, levorphanol, methadone, and fentanyl. Choice of opioid, its dose, and route of administration has to be individualized with frequent monitoring for possible side effects and risk of abuse, misuse, or diversion.
The acute sickle cell painful crisis
The acute sickle cell painful crisis is the hallmark of SCD and the number 1 cause of hospitalization.43 It is unpredictable and may be precipitated by known or unknown risk factors and triggers.44 Much of the devastation caused by the disease is the result of the recurrent acute painful crises. Clinical features of a typical painful crisis were described accurately by Diggs, that “patients experience sudden onset of pain in the low back or in one or more joints or one of the extremities. The pain may be localized or migratory and is continuous and throbbing. The severe pain causes patients to grunt, groan, cry, twist and turn and to assume abnormal postures in the futile attempt to obtain relief.”45 Descriptors of pain also include throbbing, sharp, pounding, dull, stabbing, cutting, and gnawing or like a generalized toothache.44
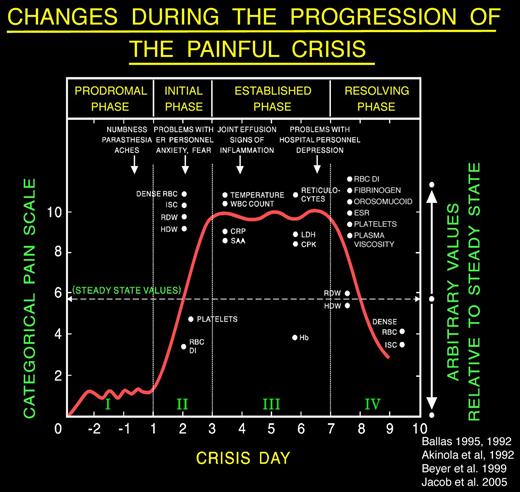
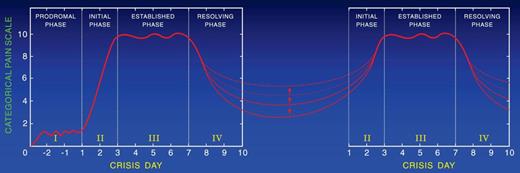
Extensive review of the literature pertinent to the clinical description of the painful crisis requiring hospitalization showed that it evolves through distinct phases. Ballas and Smith46 and Akinola et al47 independently described the presence of 2 phases of the uncomplicated painful crisis in prospective longitudinal studies of adults with SS. Akinola et al47 studied 20 patients over 16 months, and Ballas and Smith46 studied 117 painful crises affecting 36 patients with sickle cell anemia over 6 years. The initial phase was associated with increasing pain, decreased RBC deformability, increase in the number of dense cells, red cell distribution width, Hb distribution width, reticulocyte count, leukocytosis, and relative thrombocytopenia. The second phase was characterized by established pain of maximum severity and gradual reversal of the abnormalities of the first phase. Later, Ballas48 revised the description of the painful crisis and refined its evolution into 4 phases by including observation by several other investigators. For example, Murray and May reported a premonition of painful crisis suggestive of a prodromal phase of 1 to 2 days in 59 of 102 (58% patients with SCD) during a questionnaire study.49 Reported symptoms included numbness, paresthesia, or aches in the areas that subsequently became painful. The duration of the prodromal phase was up to 1 or 2 days. The phases were called prodromal, initial, established, and resolving phases (Figure 1). Beyer et al50 and Jacob et al51 reported the evolution of painful crises along similar phases in children.
A typical profile of the events that develop during the evolution of a severe sickle cell painful crisis in an adult in the absence of overt infection or other complications. Such events are usually treated in the hospital with an average stay of 9-11 days. Pain becomes most severe by day 3 of the crisis and starts decreasing by day 6 or 7. The Roman numerals refer to the phase of the crisis: I indicates prodromal phase; II, initial phase; III, established phase; and IV, resolving phase. Dots on the x-axis indicate the time when changes became apparent; and dots on the y-axis, the relative value of change compared with the steady state indicated by the horizontal dashed line. Arrows indicate the time when certain clinical signs and symptoms may become apparent. Values shown are those reported at least twice by different investigators; values that were anecdotal, unconfirmed, or that were not reported to occur on a specific day of the crisis are not shown. ISC indicates irreversibly sickled cells; RDW, red cell distribution width; HDW, hemoglobin distribution width; RBC DI, red cell deformability index; CRP, C-reactive protein; SAA, serum amyloid A; LDH, lactate dehydrogenase; CPK, creatinine phosphokinase; and ESR, erythrocyte sedimentation rate. Reproduced from Ballas48 with permission.
A typical profile of the events that develop during the evolution of a severe sickle cell painful crisis in an adult in the absence of overt infection or other complications. Such events are usually treated in the hospital with an average stay of 9-11 days. Pain becomes most severe by day 3 of the crisis and starts decreasing by day 6 or 7. The Roman numerals refer to the phase of the crisis: I indicates prodromal phase; II, initial phase; III, established phase; and IV, resolving phase. Dots on the x-axis indicate the time when changes became apparent; and dots on the y-axis, the relative value of change compared with the steady state indicated by the horizontal dashed line. Arrows indicate the time when certain clinical signs and symptoms may become apparent. Values shown are those reported at least twice by different investigators; values that were anecdotal, unconfirmed, or that were not reported to occur on a specific day of the crisis are not shown. ISC indicates irreversibly sickled cells; RDW, red cell distribution width; HDW, hemoglobin distribution width; RBC DI, red cell deformability index; CRP, C-reactive protein; SAA, serum amyloid A; LDH, lactate dehydrogenase; CPK, creatinine phosphokinase; and ESR, erythrocyte sedimentation rate. Reproduced from Ballas48 with permission.
The presence of phases of the crises allows providers to monitor the progress of the crisis and manage it according to rational basis and avoid the conflicts that often arise about the authenticity of pain. Figure 1 and Table 1 show a number of changes in objective signs during the evolution of the crisis. These changes, however, can be appreciated if the parameters are determined serially and, more importantly, if they are compared with steady-state values.52,53
Consequences of the acute painful crisis
The acute painful crisis is associated with serious complications of the disease, most frequently occurring between days 1-5. Approximately 50% of reported cases of acute chest syndrome occur after hospitalization with a painful crisis.54 Acute multiorgan failure55 and sudden death56 also have been reported during painful crises.
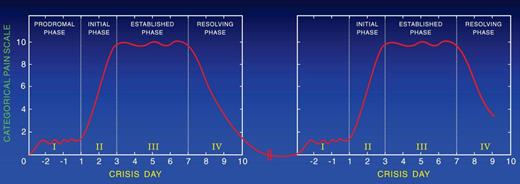
Resolution of the painful crisis is associated with rebound thrombocytosis, elevated levels of fibrinogen, orosomucoid, decreased RBC deformability, and increased plasma viscosity, all predisposing to a hypercoagulable state that may cause recurrence of the crisis (Figure 1; Table 1). To support this hypothesis, we followed patients longitudinally after discharge from the hospital to determine how often patients are readmitted after discharge. We found that ∼ 20% of the patients who were discharged from the hospital after the resolution of a crisis had recurrent crises that required treatment with parenteral opioids in the ED or hospital within 1 week after discharge (Table 2).46 A painful crisis seems to be a risk marker for a subsequent crisis; however, some patients, especially children, have a pain-free-period of variable duration before the onset of another crisis (Figure 2). Others continue to have pain. According to the Pain In Sickle Cell Epidemiology Study adult patients reported SCD pain at home on ∼ 55% of the 31 017 days,57 whereas children reported SCD pain at home on ∼ 9% of the1515 days surveyed.58,59 In MSH, at-home analgesics were used for SCD pain on 40% of diary days and 80% of 2-week follow-up periods, with short-acting oxycodone and acetaminophen being the most frequently used analgesics.21,60 Descriptors and location of pain at home were similar to those during hospitalization but milder. Importantly, at-home patients preferred to treat pain with short-acting opioids rather than the controlled-release opioids. Moreover, those who take controlled release opioids with short-acting opioids for breakthrough pain experience frequent attacks of breakthrough pain, resulting in the consumption of relatively large amounts of short-acting opioids. In addition, patients prefer to be treated in the day unit rather than the ED to avoid long hours of waiting. Treatment of patients in the day unit with parenteral short-acting opioids decreased the frequency of hospital admissions and ED visits.61
Two sequential painful crises with no pain during the time in between them.
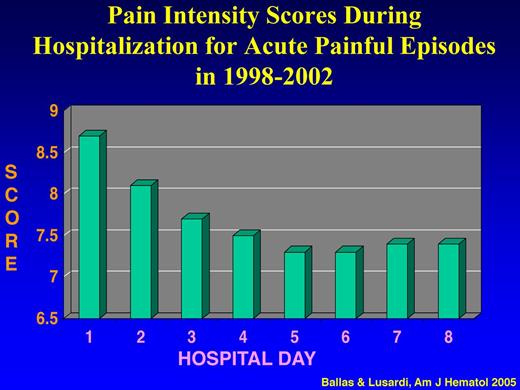
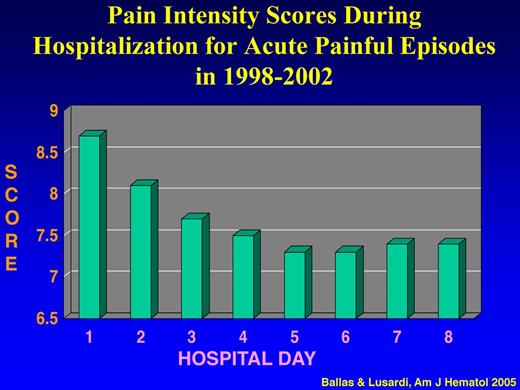
To determine the actual pattern of hospital admissions of patients with SCD and the causes of frequent hospital readmissions and their prognostic significance, a prospective longitudinal and observation cohort study of all adult patients with SS admitted to one institution between January 1998 and December 2002 was conducted.43 Analysis of the data showed that: (1) ∼ 95% of all the 1540 admissions of 136 patients were for acute painful crises; (2) the intensity of pain score decreased significantly during the first 4 days of hospital admission from an average of 8.7 ± 1.17 to 7.5 ± 1.00 (P < .001) and then reached a plateau of 7.4 until discharge (Figure 3); (3) the mean score of pain intensity was > 7 throughout the hospital stay; and (4) ∼ 50% of hospital admitted patients for acute painful episodes were readmitted within 1 month after discharge, and ∼ 16% of all admissions were readmitted within 1 week after discharge (Table 3). The major cause of hospital readmission was the same acute sickle cell pain that was not controlled with analgesics at home or in the ED. Withdrawal syndrome was the cause of readmission only in 5 patients who were readmitted collectively 46 times (∼ 7% of all readmissions) within 1 week after discharge.
Pain intensity scores during hospitalization for acute painful episodes in 1998-2002. Adapted from Ballas and Lusardi43 with permission.
Pain intensity scores during hospitalization for acute painful episodes in 1998-2002. Adapted from Ballas and Lusardi43 with permission.
Jacob et al showed similar pattern of pain score that plateaus ∼ 5 days after hospital admission for painful crises in children.62 Moreover, the high frequency of hospital readmission was confirmed by other studies.63-65 In a retrospective cohort of SCD-related ED visits and hospitalizations from 8 states in 2005 and 2006, Brousseau et al found that the 30-day and 14-day readmission rates were 33.4% and 22.1%, respectively.63 Readmissions were highest for the 18- to 30-year-old patients and for the publicly insured patients. In another retrospective study in children with SCD, the most common diagnosis for hospital admission and readmission within 30 days after discharge was acute pain.64 The major risk marker for readmission was lack of outpatient hematology follow-up within 30 days after discharge. Asthma was another risk marker for readmission within 30 days. Sobota et al identified 4762 patients with 12 104 qualifying hospitalizations of which 2074 hospitalizations (17%) were readmissions for sickle cell crisis within 30 days after discharge.65 Risk markers for readmission included older children, pain, and treatment with steroids.
Approximately 10% of children and ∼ 50% of adults with SCD continue to have pain between painful episodes. The severity and duration of each pain event between crises vary among persons. Thus, the hallmark of SCD seems to be continuous acute pain, the severity of which varies from severe to mild or moderate. When the pain is severe, it requires treatment in the ED or in the hospital with parenteral opioids; and when it is mild or moderate, it is treated at home with oral analgesics. The transition from acute painful crises with no pain in between to acute painful crises with mild or moderate pain in between is age dependent. Thus, the majority of children are pain free between recurrent painful episodes (Figure 2) and ∼ 50% of adults continue to have mild to moderate pain between relapsing crises (Figure 4). The opinion of 2 of the authors (S.K.B. and P.A.-G.) based on their experience is that descriptors of the pain are mostly the same as in acute crisis and that short-acting opioids are preferred for analgesia by the majority of adult patients. These opinions are further supported by a follow-up study of MSH showing that at home analgesics were used on 40% of diary days and 80% of 2-week follow-up periods, with oxycodone and codeine the most frequently used analgesics.21
Two sequential painful crises with residual pain of variable severity during the time in between them.
Two sequential painful crises with residual pain of variable severity during the time in between them.
Pathophysiology of acute pain
The pathophysiology of acute pain in general has been extensively reviewed in major textbooks on pain medicine.66-68 The reader is advised to consult these references for more details. This section will discuss the highlights of this subject.
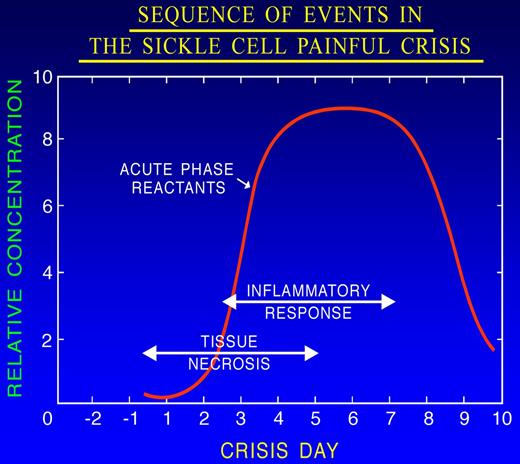
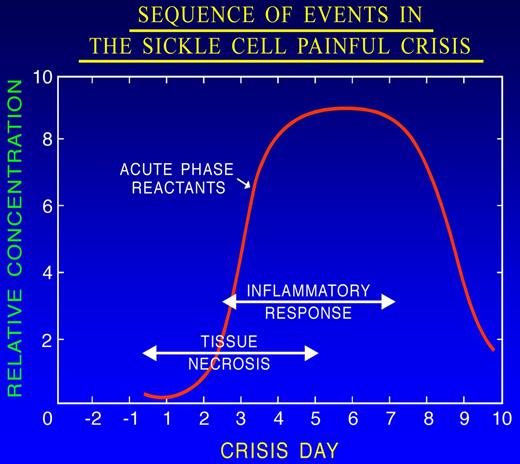
Vaso-occlusion leads to hypoxia, ischemia, and eventually tissue damage followed by chronic vascular inflammation, underlying many features of sickle cell pain.69-71 Inflammatory mediators released from injured cells, macrophages, mast cells, and platelets66,67 activate nociceptors on the peripheral afferent, thus initiating the nociceptive insult. It is the combination of hypoxia/reperfusion injury, ischemic tissue damage, and inflammation that makes the pain of SCD unique (Figure 5). Indeed, hypoxia/reoxygenation simulating vaso-occlusive crisis increased pain in transgenic sickle mice.72
Sequence of events during the evolution of the prodromal phase of the painful crisis. Tissue necrosis consequent to ischemia elicits an inflammatory response that is associated with an increase in the serum level of acute phase reactants. Adapted from Ballas101 with permission.
Sequence of events during the evolution of the prodromal phase of the painful crisis. Tissue necrosis consequent to ischemia elicits an inflammatory response that is associated with an increase in the serum level of acute phase reactants. Adapted from Ballas101 with permission.
Peripheral nerve endings have multiple receptors that include various families of proteins that respond to specific molecules such as ATP and H+ ions. The transient receptor potential vanilloid type 1(TRPV1) has recently received special attention in pain medicine because its activation is associated with the sensation of pain.67 Activation of receptors with glutamate as the neurotransmitter transforms chemical, mechanical, or thermal energy into electrical impulse transmitted along Aδ and C fibers to the dorsal horn of the spinal cord via the dorsal root ganglion. At this junction, connections with the autonomic nervous system occur and substance P, calcitonin gene related peptide, and norepinephrine are released antidromically to the periphery, thus creating a vicious circle. Complex series of biochemical events occur at the level of laminae I-VI of the dorsal horn of the spinal cord resulting in facilitation or inhibition of the transmission of the painful stimuli. Proinflammatory states and impaired pain regulation, for example, are factors that may contribute to pain amplification.73 From the dorsal horn of the spinal cord, the electrical impulse of pain ascends along the contralateral spinothalamic tract to the thalamus that interconnects reversibly with other centers, most notably the limbic system (mediator of memory and emotion), the reward system (mediator of pleasure and addiction), and the glia. The pathway of pain stimuli is subject not only to activators, sensitizers, and facilitators, but also to inhibitors. Serotonin, norepinephrine, enkephalin, β-endorphin, and dynorphin are endogenous central pain inhibitors. The net outcome of tissue ischemia may be severe or mild pain, depending on the extent of tissue damage and the net balance of pain stimulators versus pain inhibitors. This may explain, in part, the considerable variation in the frequency and severity of painful sickle crises among patients. Moreover, psychological, social, cultural, and spiritual factors often unite and conspire with vaso-occlusion to initiate the unique nature of sickle cell pain.
Central sensitization and neuroplasticity
Central sensitization is the process by which excessive nociceptive signals bombarding the CNS from the periphery cause changes both in the spinal cord and the brain that result in continuous amplification of pain sensation.74-76 Clinical manifestations of central sensitization include: (1) reduced pain threshold resulting in hyperalgesia (exaggerated response to normally mild painful stimuli) and allodynia (a phenomenon where innocuous ambient stimuli become painful); (2) expanded receptive fields, which refers to hyperalgesia occurring beyond the area of original injury; and (3) after sensations where pain sensation continues after cessation of the original injury.
Another type of modification of the repetitive and severe painful impulse occurs at the level of the brain.76,77 The main function of the brain is message transmission. To do this, it has 100 billion neurons and each neuron connects to 10 000 other neurons via synapses resulting in 1 million billion connections.78 Thus, the severe and repetitive pain impulse that reaches the brain excites an enormous number of neurons that stimulate other neurons in a progressive manner, resulting in the perception of severe pain. Neuroscientists describe this mass transit of information as “cells that fire together wire together.”78 Moreover, the brain is neuroplastic where nociceptive input from the periphery causes functional and structural changes that may result in altered perceptual processing and continuity of pain.78 This is often referred to as “rewiring” of the brain in patients who have persistent severe pain. Alternative circuits of pain transmission and remapping of functional connections78 occur in the brains of these patients. The best example of this phenomenon in patients with SCD is that severe pain persisted for several weeks or months, even in those patients who were cured of sickle cell anemia after successful bone marrow transplantation.79 It takes a relatively long time to reset the “wiring” of the brain back to normal in these patients. Another possible explanation for this delay in pain resolution is that it took this long for target sites to heal its regional tissue damage, blood vessel damage, or peripheral nerve damage.
Chronic pain
Chronic pain is often defined as pain that persists for 3 or more months. Sources of chronic pain in SCD include bone infarction, avascular necrosis of joints, back pain from disk protrusion into vertebral bodies, leg ulcers, and chronic osteomyelitis.80 The descriptors of the pain of these conditions are different from the pain associated with the acute painful crisis or the pain that persists between crises. The pain of these syndromes is usually treated with long-acting or control-release opioids with less use of short-acting opioids for breakthrough pain in contrast to the case in the persistent pain between acute painful crises.81 Moreover, these pain syndromes rarely require hospital admission, except when a painful crisis is superimposed on the chronic pain or if the underlying condition is associated with severe infection that requires parenteral antibiotics as may happen in leg ulcers.
We and others previously described a second type of chronic pain in SCD.80,82-84 It is intractable chronic pain without obvious pathology where the only complaint is the patient's self-report of pain that does not go away. We think we were adopting and borrowing concepts from other pain disciplines. We need to reconsider this. SCD does have obvious pathology documented by the presence of the sickle gene and sickle Hb, which are pathologic. It seems we were referring to the persistent pain between crises as chronic pain without pathology.
Neuropathic pain
Neuropathic pain is usually described as numb, tingling, lancinating, spontaneous, shooting, or paroxysmal in nature associated with a sensation of pins and needles, hyperalgesia, and allodynia (pain resulting from ambient non-noxious stimuli).80,83 Its severity is also enhanced by exposure to either cold or heat. This pain could be secondary to nerve injury or nerve dysfunction whether peripherally or centrally. Anecdotally, neuropathic pain in SCD may be the result of tissue damage after vaso-occlusion of blood vessels of nerves (vasa vasorum). These include mental nerve neuropathy,85-88 trigeminal neuralgia,89 acute proximal median mononeuropathy,90 entrapment neuropathy,91 acute demyelinating polyneuropathy,91 ischemic optic neuropathy,92 orbital infarction,93 orbital apex syndrome,94 and spinal cord infarction.95 Among the peripheral neuropathic pain syndromes in SCD, mental nerve neuropathy was the most commonly reported. Clinically, it is characterized by numbness of the chin with mandibular bone pain speculated to result from nerve ischemia or compression because of mandibular bone infarction. Most of the reported cases occurred during painful sickle crises and resolved after the resolution of the crisis. It is more common in females and was bilateral in 1 patient. Neuropathic pain that is often associated with persistent acute or chronic pain has not been well studied in patients with SCD to date.
Sickle cell pain in mice expressing sickle Hb
Although neuropathic pain in patients with SCD is not well understood, its presence in the transgenic sickle mouse may offer insight. Mice expressing sickle Hb exhibited pain characteristics similar to those observed in patients with SCD.72,96 Transgenic mice expressing various levels of sickle Hb demonstrated increased sensitivity to cold, heat, and mechanical stimuli and to deep tissue/musculoskeletal pain compared with their age-matched controls. Hypoxia/reoxygenation further increased pain behaviors in these sickle Hb-expressing mice.72 Activators of neuropathic and inflammatory pain, including phospho-p38MAPK, phospho-MAPK/ERK, phospho-STAT3, COX-2, and toll-like receptor 4, were markedly increased in the spinal cord of sickle Hb-expressing mice compared with control mice expressing normal human Hb. Thus, neurochemical changes in the skin and molecular alterations in the spinal cord in association with alteration in the peripheral nerve fibers in the skin support the existence of inflammatory and neuropathic pain in sickle mice.
TRPV1 channels are nonselective cation channels that mediate thermal and mechanical hyperalgesia in response to noxious stimuli, such as heat, inflammation, and nerve injury.97,98 Hillery et al observed that TRPV1 channel was functionally activated in the primary afferents in skin-nerve preparations and in the somata of isolated DRG of BERK sickle mice with increased mechanical sensitization.98 Mechanical sensitivity persisted in somata even 36 hours after isolation, suggestive of nociceptor sensitization in sickle mice. A TRPV1 channel antagonist A-425619 completely reversed the nociceptor sensitization and partially blocked the behavioral hypersensitivity to mechanical stimuli in sickle mice. Thus, TRPV1 channel activation may contribute to chronic and acute pain in SCD. Substance P is known to sensitize TRPV1 channels in peripheral nociceptors.99 Elevated levels of substance P in the serum of patients with SCD and increased expression of substance P in the skin of sickle mice support the existence of nociceptor sensitization in SCD.
Redefining sickle cell pain
Although the molecular pathophysiology of SCD is well understood, the management of sickle cell pain, its hallmark, is an embarrassing failure. Our perspectives redefine sickle cell pain and its unique features based on available data and the experience of experts in the field that has accumulated since 1910. This redefinition may reveal new avenues to improve our understanding and management of this complex pain syndrome. Our knowledge of sickle cell pain that evolved over the last 102 years suggests that the hallmark of SCD is acute pain that waxes and wanes, relapses, and remits in a recurrent and unpredictable fashion. When severe, it is the acute painful crisis that requires treatment with parenteral analgesics in the ED or hospital. When mild or moderated, it is the residual or persistent acute pain between 2 subsequent acute painful episodes. This explains why patients are often readmitted after discharge, why they prefer using short-acting analgesics, and why they are treated in the day unit or ED frequently with short-acting opioids between hospital admissions. The use of “chronic sickle cell pain” should be limited to the chronic pain syndromes mentioned in “Chronic pain.”
Pain-free intervals are longer for younger than for older patients with SCD. Pain-free intervals shorten with aging. Figure 2 shows the pattern of pain in patients who are pain-free between crises, and Figure 4 shows the pattern in patients who continue to have pain between crises. The severity of the residual pain between crises varies in intensity among patients and in the same patient with time. The pattern of pain shown in Figure 4 occurs in ∼ 50% of adult patients with SCD.21,57,60
New approach to therapy
Our hypothesis is that acute painful crisis treated aggressively at its onset; its outcome would be of shorter duration with fewer complications. Unfortunately, the end of an acute sickle cell painful crisis at the present is a fuzzy point in time. Discharge from the ED or hospital does not necessarily indicate the end of the crisis. It is an arbitrary point that depends on several factors that are not based on the individual status of the patient but subscribes to insurance coverage, hospital policy, and the attitude of the providers. Table 4 lists the approaches for the treatment of SCD and its complications.100 The ultimate goal is to achieve a cure. Short of that, preventing the painful crisis with agents that induce the production of Hb F is possible; and short of that, aborting the painful crisis is highly desirable. A triad of pathophysiologic factors initiates the acute painful crisis: vaso-occlusion, inflammation, and nociception. Each painful crisis is associated with residual inflammatory damage that accumulates with recurrent crises culminating in organ dysfunction and organ failure. The rational approach to abort a crisis is to treat it as early as possible, preferably at the prodromal phase, where tissue ischemia and inflammation are in their early stages101 (Figure 5). The goal of management at this phase is a trial of anti–vaso-occlusive therapy akin to therapy of ischemic stroke in adults in the general population102,103 and to the treatment of acute myocardial infarction.104,105 The standard of care of ischemic stroke and myocardial infarction is to give tissue plasminogen activator, a protein that breaks down thrombi or clots, within a few hours after the onset of signs and symptoms to restore perfusion. Beyond that, tissue plasminogen activator will not be as effective. Anti–vaso-occlusive therapy to abort a crisis at the prodromal stage should include a trial of hydration, anti-inflammatories, analgesics, and, possibly, vasodilators. Patients with SCD could be counseled and trained to treat themselves at home as soon as they feel an impending crisis. Nitric oxide (NO) would be the ideal vasodilator for self-administration at home. Gladwin et al showed that NO in the hospital is not effective in reducing the hospital length of stay of patients with painful crises.106 Administration of NO in the ED, however, was effective in aborting crisis in some patients.107,108 It is probable that shifting the use of NO to the left (at home) rather than to the right (hospital) is the right way to go. Moreover, blood transfusion or vigorous hydration at the prodromal phase may be effective in aborting the crisis. Sobota et al reported that transfused pediatric patients with crisis were readmitted less frequently to the hospital than otherwise.65 The combination of NO and blood transfusion/hydration in the prodromal phase would restore perfusion and prevent or minimize damage because of hypoxia, the addition of anti-inflammatories would prevent or minimize damage because of inflammation, and, most importantly, aggressive management of pain may prevent central sensitization and “rewiring” of the brain.
Currently, patients with acute painful crisis receive treatment ∼ 2-3 days after the onset of the early prodromal signs and symptoms of the crisis. By that time, irreversible tissue damage is well established and difficult to reverse. This has to change. The rational therapy of acute sickle cell pain must be actively based on its own mechanistic pathophysiology and not by the passive adoption of guidelines of other pain syndromes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alden Blake Daniels, an undergraduate student at the University of Memphis, for his support in collecting data published by Drs Diggs and Kraus since 1930, and Bernice Fenner for administrative assistance.
Authorship
Contribution: S.K.B. designed and wrote the manuscript; K.G. wrote the section that pertains to pain and the systemic effects of morphine in the transgenic sickle cell mouse; P.A.G. reviewed and summarized data collected and published by Drs Diggs and Kraus since 1930; and all authors contributed comments and revised the manuscript for critical content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samir K. Ballas, Cardeza Foundation, 1015 Walnut St, Philadelphia, PA 19107; e-mail samir.ballas@jefferson.edu.