Abstract
Patients with β-thalassemia major (TM) and other refractory anemias requiring regular blood transfusions accumulate iron that damages the liver, endocrine system, and most importantly the heart. The prognosis in TM has improved remarkably over the past 10 years. This improvement has resulted from the development of magnetic resonance imaging (MRI) techniques, especially T2*, to accurately measure cardiac and liver iron, and from the availability of 3 iron-chelating drugs. In this article we describe the use of MRI to determine which adult and pediatric patients need to begin iron chelation therapy and to monitor their progress. We summarize the properties of each of the 3 drugs, deferoxamine (DFO), deferiprone (DFP), and deferasirox (DFX), including their efficacy, patient acceptability, and side effects. We describe when to initiate or intensify therapy, switch to another drug, or use combined therapy. We also discuss the management of refractory anemias other than TM that may require multiple blood transfusions, including sickle cell anemia and myelodysplasia. The development of a potential fourth chelator FBS 0701 and the combined use of oral chelators may further improve the quality of life and survival in patients with TM and other transfusion-dependent patients.
Introduction
Iron overload is a major concern in patients with congenital and acquired anemias for whom regular transfusions are needed (Table 1). Under normal conditions, iron absorption and loss are balanced at ∼ 1 mg/day. Transfused blood contains 200-250 mg of iron per unit. Hence, patients with β-thalassemia major (TM) or other refractory anemias receiving 2-4 units of blood per month have an annual intake of 5000-10 000 mg of iron or 0.3-0.6 mg/kg per day. The body has no mechanism for excreting this excess iron. Moreover, patients with TM and other anemias characterized by ineffective erythropoiesis absorb excess iron despite iron overload because of production of GDF15 and possibly other proteins (eg, TWSGI) from erythroblasts, which inhibit hepcidin synthesis.1
Refractory anemias for which blood transfusions and iron chelation may be needed
| Congenital . | Acquired . |
|---|---|
| TM | Aplastic anemia |
| Thalassemia intermedia | Red cell aplasia |
| Aplastic anemia (Fanconi) | Myelodysplasia |
| Blackfan-Diamond anemia | Chronic myelofibrosis |
| Sideroblastic anemia | Paroxysmal nocturnal hemoglobinuria |
| Sickle cell anemia | |
| Rarely in some cases of congenital hemolytic anemia (eg, pyruvate kinase, glucose-6-phosphate dehydrogenase deficiency) |
| Congenital . | Acquired . |
|---|---|
| TM | Aplastic anemia |
| Thalassemia intermedia | Red cell aplasia |
| Aplastic anemia (Fanconi) | Myelodysplasia |
| Blackfan-Diamond anemia | Chronic myelofibrosis |
| Sideroblastic anemia | Paroxysmal nocturnal hemoglobinuria |
| Sickle cell anemia | |
| Rarely in some cases of congenital hemolytic anemia (eg, pyruvate kinase, glucose-6-phosphate dehydrogenase deficiency) |
Untreated transfusional iron load results in damage to the liver, endocrine organs, and most importantly to the heart. In TM, without effective iron chelation, death occurs from cardiac failure or arrhythmia, usually in late childhood or in the teenage years. Most studies of iron chelation therapy have been carried out in TM for which all patients need transfusions and iron chelation. As discussed in “Congenital anemias” and “Acquired anemias,” the exact indication for iron chelation and the cost/benefit is much less well established for patients with sickle cell disease (SCD), myelodysplasia (MDS), and other refractory anemias.
We first highlight the available techniques used for assessing iron status. We then review the efficacy, side effects, and how we monitor treatment with the 3 currently licensed iron chelators deferoxamine (DFO), deferiprone (DFP), and deferasirox (DFX) alone or in combination. We then describe how we commence iron chelation in adults and children with TM and transfusional iron overload in conditions other than TM. The overall management of TM has already been superbly reviewed in this series of How I Treat,2 and recent excellent reviews of iron chelation therapy have been published.3,4
Assessment of iron overload
Calculation of iron intake recording the number of units of blood transfused is cost-effective and precise and can predict the total iron that will accumulate in the body.
Serum ferritin
Serum ferritin measurement may be the only available method of assessing iron burden in developing countries. It is useful for close and frequent patient monitoring to indicate changes in iron burden. More accurate measurements of iron stores (see next section) are performed at less frequent intervals. Although serum ferritin has been used for deciding when to start chelation therapy, it is now known to be an inaccurate indicator of cardiac iron or of total body iron burden. Serum ferritin also fluctuates in response to inflammation, abnormal liver function, and ascorbate deficiency. Despite these reservations, there is an association, albeit weak, between the level of serum ferritin and prognosis in TM.8-12
LIC
Liver iron concentration (LIC) accurately predicts total body iron stores.13 When possible, it should be measured annually in patients undergoing regular transfusion therapy. Normal LIC values are up to 1.8 mg Fe/g dry weight, with levels of up to 7 mg/g dry weight seen in carriers of genetic hemochromatosis without apparent adverse effects. Several studies have linked very high LIC (> 15 mg/g dry weight) to worsening prognosis,10,14 liver fibrosis progression,15 and liver function abnormalities.16 It is likely that very high liver iron concentrations are associated with high plasma non-transferrin bound iron (NTBI) because the liver is the main organ for removing free iron from plasma. NTBI is damaging to the organs that are also affected by iron deposition.
Liver biopsy provides a direct measurement of LIC, being quantitative, specific, and sensitive. Biopsy is an invasive procedure, but in experienced hands it has a low complication rate.15 Inadequate sample size (< 1 mg/g dry weight or < 4 mg wet weight or < 2.5 cm core length) or uneven distribution of iron, particularly in the presence of cirrhosis, may give misleading results.17 LIC can also be measured accurately by superconducting quantum interference device. However, only 4 such machines are available worldwide: they are expensive to purchase and maintain and require dedicated trained staff. The results correlate well with chemical estimation of LIC unless fibrosis is present.
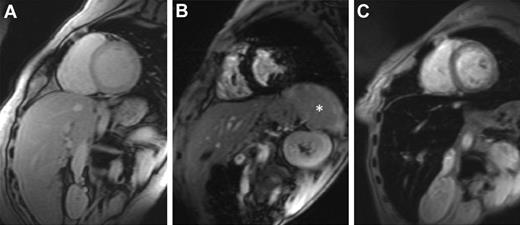
MRI is more widely available, and it offers noninvasive estimation of LIC. MRI scanners generate images of organs in which the signal seen depends on iron concentration. Iron causes the organ to darken more rapidly (Figure 1). T2* is the time needed for the organ to lose approximately two-thirds of its signal and is measured in milliseconds (ms). T2* shortens as iron concentration increases. Its reciprocal, 1000/T2*, is known as R2* and is measured in units of inverse seconds (S−1).
Cardiovascular magnetic resonance T2* images showing the heart and liver from 3 different patients at the same echo time (10.68 ms). (A) Normal appearance with a bright myocardial and liver signal indicating that there is no significant cardiac or hepatic iron loading (myocardial T2* 29 ms, liver T2* 22 ms). (B) Dark myocardial signal indicating severe myocardial siderosis (heart T2* 6.2 ms) but no liver iron (liver T2* 18 ms). *The spleen also has high signal, suggesting that there is no significant splenic iron loading. (C) Normal myocardial signal (heart T2* 24 ms) but dark liver consistent with severe hepatic iron overload (liver T2* 1.8 ms). Images courtesy of Dr J. P. Carpenter (The Royal Brompton Hospital, London, United Kingdom).
Cardiovascular magnetic resonance T2* images showing the heart and liver from 3 different patients at the same echo time (10.68 ms). (A) Normal appearance with a bright myocardial and liver signal indicating that there is no significant cardiac or hepatic iron loading (myocardial T2* 29 ms, liver T2* 22 ms). (B) Dark myocardial signal indicating severe myocardial siderosis (heart T2* 6.2 ms) but no liver iron (liver T2* 18 ms). *The spleen also has high signal, suggesting that there is no significant splenic iron loading. (C) Normal myocardial signal (heart T2* 24 ms) but dark liver consistent with severe hepatic iron overload (liver T2* 1.8 ms). Images courtesy of Dr J. P. Carpenter (The Royal Brompton Hospital, London, United Kingdom).
MRI scanners can also measure T2 and R2 rather than T2* and R2*, although technically this is slower and less straight forward. The results of R2* and R2 for liver iron are similar.18 The technique demonstrates an average sensitivity of > 85% and specificity of > 92% up to an LIC of 15 mg/g dry weight and has been registered in the European Union and the United States. For calibration, the MRI machine must use a Phantom supplied by the company, whereas the data acquired are sent via internet for analysis by the dedicated FerriScan ISO 13285 accredited analysis facility (payment per scan analyzed). It can be applied with little training, at any center with an up-to-date MRI machine. T2* MRI for liver iron quantification is also widely used. Liver T2* calibration using a clinically relevant MRI sequence has been published.18 T2* measurement of LIC is reproducible between centers using a clinical-grade MRI sequence.19 We prefer the T2* technique to T2 as it offers measurement of both cardiac and hepatic iron overload at the same time (see next section). T2 and R2 for measuring cardiac iron are less robust but widely used.
Cardiac iron
Estimation of myocardial iron using T2* MRI requires expertise in its use and standardization. Good correlation between different centers and machines has been shown,19 and the technique has been recently validated as a true measure of cardiac iron, correlating with chemical measurement on postmortem cardiac biopsies.20 A shortening of myocardial T2* to < 20 ms (implying increased myocardial iron above normal) is associated with an increased likelihood of decreased left ventricular ejection fraction (LVEF), whereas patients with T2* values > 20 ms have a very low likelihood of decreased LVEF.4 T2* values of 10-20 ms indicate a 10% chance of decreased LVEF; 8-10 ms an 18% chance; 6 ms a 38% chance; and T2* values of 4 ms a 70% chance of decreased LVEF.7
Cardiac T2* therefore identifies those patients at risk of a fall in LVEF whose chelation treatment should be intensified.5,12,21,22 Improved survival in patients with TM in the United Kingdom has been attributed to the introduction of cardiac MRI T2* monitoring with intensification of chelation if indicated as well as the availability of the oral iron chelator DFP.23
Cardiac T2* does not correlate with serum ferritin concentration or liver T2* in patients receiving chelation therapy in a cross-sectional analysis, although longitudinal studies may imply a significant relationship.4-6 The discrepancy between cardiac iron and LIC in many TM patients may be partly the result of the differences in response to DFO therapy, which removes liver iron more effectively than cardiac iron.24,25 However, even in the absence of DFO therapy, TM patients may develop a cardiac T2* < 20 ms with LIC concentrations in the range of 1.2-9.0 mg/g dry weight.26 Thus, cardiac MRI T2* measurement is needed in all TM patients irrespective of their LIC or serum ferritin level. We recommend that patients undergo cardiac MRI T2* measurement at least yearly if they have abnormal values (< 20 ms) or more frequently if with diagnosed heart disease; and once every 2 years in those with values > 20 ms and normal cardiac function. All patients should have cardiac T2* measured if cardiac symptoms develop.
Other measurements
Other tests of iron status cannot be recommended for regular monitoring of iron overload or response to chelation therapy. Measurement of NTBI is carried out only in a few research laboratories. Urine iron excretion after a single dose of a chelator gives some measure of total iron stores in the case of DFP but not for DFX or DFO where iron excretion is totally or partly by the fecal route. Measurement of the degree of saturation of the plasma iron binding capacity gives a rough idea of iron burden but is affected by recent iron chelation therapy or inflammation. Values > 100%, however, suggest inadequate chelation and the need for cardiac and liver iron determination.
Available chelators
DFO.
DFO is the drug for which there is the longest experience in treating transfusional iron overload (Table 2). It is usually self-infused on at least 4 days a week over 8-12 hours. The usual dose is 40 mg/kg body weight, but higher doses up to 60 mg/kg have been used in patients with high body iron stores. Even higher doses have caused pulmonary and neurotoxicity and should be avoided. Vitamin C (maximum 200 mg daily) may be given to correct deficiency and to enhance iron excretion. Some units give intravenous DFO (from a separate bag) with blood transfusion (eg, 1 g of DFO for each unit of blood), but we do not recommend this in children or in adults unless noncompliant and inadequately chelated.
Comparison of DFO, DFP, and DFX
| . | DFO (DFO) . | DFP (DFP) . | DFX (DFX) . |
|---|---|---|---|
| Molecular weight | 560 | 139 | 373 |
| Chelator: iron | 1:1 (hexandentate) | 3:1 (bidentate) | 2:1 (tridentate) |
| Route of administration | Subcutaneous or intravenous | Oral tablets or liquid | Oral suspension |
| Iron excretion | Urine, fecal | Urine | Fecal |
| Plasma half-life | 20 min | 1-3 h | 8-16 h |
| Usual dose | 40 mg/kg/d | 75-100 mg/kg/d | 20-40 mg/kg/d |
| Licensed | Licensed for treatment of chronic iron overload resulting from transfusion-dependent anemia | In Europe, North America, and Asia: for treatment of iron overload in TM where DFO is contraindicated or inadequate | In the United States, licensed for treatment of transfusional iron overload in patients 2 years or older. In Europe, approved for treatment of transfusional iron overload in TM, 6 years and older and when DFO is contraindicated and inadequate, in patients with other anemias, patients 2-5 years old and in nontransfusion-dependent thalassemia |
| Cardiac iron removal | Compliance problem; not effective in all compliant patients; continuous infusion more effective | Most effective of the 3 chelators; used with continuous DFO in cardiac failure | Reduces LIC and improves liver pathology; reduces cardiac iron in 3-year study |
| Annual cost (54 kg body weight) | 40 mg/kg/5 d = £4788* | 75 mg/kg/d = £4505 | 20 mg/kg/d = £13 245 |
| (United Kingdom NHS) | 100 mg/kg/d = £6007 | 30 mg/kg/d = £19 865 | |
| Not applicable at the same rate in all countries | 40 mg/kg/d = £26 490 | ||
| Main side effects | Local reactions, auditory, retina, allergy, bone abnormalities, Yersinia infection | Gastrointestinal, neutropenia/ agranulocytosis, arthralgia, liver enzyme rise, zinc deficiency† | Gastrointestinal, rash, renal, liver† |
| Advantages | 36 years of experience | Best for cardiac iron removal | Once-daily administration |
| Disadvantages | Mode of administration, lack of compliance | Weekly blood count monitoring in first year | Cost |
| . | DFO (DFO) . | DFP (DFP) . | DFX (DFX) . |
|---|---|---|---|
| Molecular weight | 560 | 139 | 373 |
| Chelator: iron | 1:1 (hexandentate) | 3:1 (bidentate) | 2:1 (tridentate) |
| Route of administration | Subcutaneous or intravenous | Oral tablets or liquid | Oral suspension |
| Iron excretion | Urine, fecal | Urine | Fecal |
| Plasma half-life | 20 min | 1-3 h | 8-16 h |
| Usual dose | 40 mg/kg/d | 75-100 mg/kg/d | 20-40 mg/kg/d |
| Licensed | Licensed for treatment of chronic iron overload resulting from transfusion-dependent anemia | In Europe, North America, and Asia: for treatment of iron overload in TM where DFO is contraindicated or inadequate | In the United States, licensed for treatment of transfusional iron overload in patients 2 years or older. In Europe, approved for treatment of transfusional iron overload in TM, 6 years and older and when DFO is contraindicated and inadequate, in patients with other anemias, patients 2-5 years old and in nontransfusion-dependent thalassemia |
| Cardiac iron removal | Compliance problem; not effective in all compliant patients; continuous infusion more effective | Most effective of the 3 chelators; used with continuous DFO in cardiac failure | Reduces LIC and improves liver pathology; reduces cardiac iron in 3-year study |
| Annual cost (54 kg body weight) | 40 mg/kg/5 d = £4788* | 75 mg/kg/d = £4505 | 20 mg/kg/d = £13 245 |
| (United Kingdom NHS) | 100 mg/kg/d = £6007 | 30 mg/kg/d = £19 865 | |
| Not applicable at the same rate in all countries | 40 mg/kg/d = £26 490 | ||
| Main side effects | Local reactions, auditory, retina, allergy, bone abnormalities, Yersinia infection | Gastrointestinal, neutropenia/ agranulocytosis, arthralgia, liver enzyme rise, zinc deficiency† | Gastrointestinal, rash, renal, liver† |
| Advantages | 36 years of experience | Best for cardiac iron removal | Once-daily administration |
| Disadvantages | Mode of administration, lack of compliance | Weekly blood count monitoring in first year | Cost |
The drug has transformed life expectancy for many patients with TM and other refractory anemias. It has also reduced endocrine and hepatic complications. Many patients with TM are not satisfactorily chelated by it, however, and then may develop a fatal cardiomyopathy. The reasons for these “failures” of DFO therapy include cost of the drug, pump and tubing, poor compliance,8 allergy, toxicity, local problems at the site of the infusions, lack of 24-hour binding of NTBI,27 and Yersinia infection (not a complication of the oral chelators). Even among patients apparently complying with DFO infusions at least 5 times a week and with serum ferritin levels < 1000 μg/L, some may develop cardiac iron overload and failure.28 Approximately 20% of patients receiving DFO alone in the United Kingdom, Italy, and Cyprus have cardiac T2* levels < 10 ms.29
Safety monitoring.
This has been the subject of several excellent reviews and is only briefly discussed here.3,30,31 The main side effects occur with high doses of the drug in patients, particularly children, with low iron stores. These consist of damage to the retina (night blindness, visual field loss, retinal pigmentation, and changes on electrical tests) and high tone sensory neural hearing loss. Growth and bone defects may also occur in children, with rickets-like bone lesions, metaphyseal changes, and spinal damage with loss of sitting height. A therapeutic index can be calculated as follows: mean daily dose (mg/kg)/current serum ferritin (μg/L). If this is < 0.025 at all times, these side effects of DFO do not occur.32
Regular checks are needed for visual or auditory defects, in children every 6 months and in adults annually. In children, checks of growth, particularly sitting height compared with total height, detect early spinal growth defects.
DFP (L1, 1,2 dimethyl, 3 hydroxy, pyrid-4-one, Ferriprox).
This bidentate iron chelator is rapidly absorbed with a peak blood level about 45 minutes after ingestion. It is cleared rapidly from plasma with 85% conversion in the liver to a glucuronide derivative. Differences in the speed of this conversion partly accounts for a variation in efficacy between patients.33 It is usually given 3 times daily to achieve maximum iron chelation. The usual starting dose is 75 mg/kg per day but, provided it is well tolerated, doses up to 100 mg/kg per day can be given to enhance iron excretion.34,35 Patient compliance is excellent compared with DFO.33
DFP has the lowest molecular weight of the 3 chelators and penetrates cells to chelate iron from intracellular compartments, such as lysosomes and mitochondria.36 DFP has emerged as superior to DFO at reducing cardiac iron levels. Comparison of 359 Italian patients attending 7 centers between the years 1995-2003, treated with DFO alone or for the 157 switched to DFP showed a clear superiority of DFP. In Italy, whereas no cardiac deaths and no new cardiac events (arrhythmias or cardiac failure needing drug therapy) occurred in a DFP group, 10 cardiac deaths and 42 nonfatal cardiac events occurred in a DFO group.37 Four retrospective studies in which cardiac iron in TM patients was assessed by T2* MRI suggested that DFP was more effective than DFO at removing cardiac iron. The patients receiving DFP had higher LVEFs than those receiving DFO, although for liver iron, the 2 drugs appeared equally effective.38-41
Prospective randomized trials have confirmed the superiority of DFP alone compared with DFO at usual therapeutic doses, at removing cardiac iron, improving left ventricular function, and preventing death.42,43 This was shown initially for patients with normal cardiac function and moderate cardiac siderosis (T2* 8-20 ms).42 In a subsequent large observational study, DFP improved all degrees of cardiac iron burden, including those with T2* < 8 ms.43 In this study, DFP also seemed superior to DFX at lowering cardiac iron, although nonprospective limited data were included.
Safety monitoring.
The established side effects of deferiprone were described within 2 years of the first clinical trials33 and their incidence determined in large clinical trials43-45 (Table 3). The most frequent are gastrointestinal, such as nausea, vomiting, and abdominal pain. A new liquid formulation has been reported to give fewer gastrointestinal adverse reactions.46
DFP- and DFX-related adverse effects and their management
| Adverse events . | Incidence in core trials, % . | Monitoring and management . |
|---|---|---|
| DFP-related | ||
| Gastrointestinal (nausea, vomiting, and abdominal pain) | 33 in first y | Mild: continue drug or reduce dose. If severe, discontinue drug temporarily and restart at lower dose. Try liquid formation. 5% of patients discontinue drug permanently. |
| Neutropenia (neutrophils < 1.5 × 109/L) | 8.5 | Monitor blood count weekly for first year, fortnightly in second and subsequent years. Stop drugs for a few weeks. Rechallenge and continue drug if neutrophils at safe level of > 1.5 × 109/L. |
| Agranulocytosis (neutrophils < 0.5 × 109/L) | 1 | Stop drug, treat with intravenous antibiotics if febrile. Give G-CSF if neutropenia prolonged and/or febrile. |
| Rise in transaminases | 7 | Continue to monitor without stopping drug. Enzymes usually fall to normal. If persistently raised > 2 times ULN ( ∼ 1% of patients), discontinue drug. |
| Arthropathy | 3.9-41 | Related to degree of iron overload in some series. Stop drug temporarily and restart at lower dose. ∼ 2% of patients stop drug permanently because of arthropathy. |
| Zinc deficiency | Rare: mainly in diabetes | Give zinc supplements. |
| DFX-related* | ||
| Gastrointestinal | ||
| Diarrhea | 8.8 | Patients should take an antidiarrheal for up to 2 days and keep hydrated. DFX could be taken in the evening rather than the morning. Products, such as Lactaid (if the patient is lactose intolerant) or probiotics (acidophilus or lactobacillus), could be added to the diet. |
| Abdominal pain | 5.0 | Patients should sip water or other clear fluids, and avoid solid food for the first few hours. Avoid narcotic pain medications and nonsteroidal anti-inflammatory drugs. DFX could be taken in the evening rather than the morning. |
| Nausea/vomiting | 14.3 | Patients should drink small, steady amounts of clear liquids, such as electrolyte solutions, and keep hydrated. |
| Skin rash | ||
| Mild to moderate | 4.3 | Likely to resolve spontaneously. DFX should be continued without dose adjustment. |
| Severe | 0.4 | DFX should be interrupted and reintroduced at a lower dose. Patients should take low-dose oral steroids for a short period of time. |
| Renal changes | 36 | Serum creatinine levels should be assessed in duplicate before therapy, then monthly. If patients have additional renal risk factors, serum creatinine levels should be monitored weekly for the first month or after modification of DFX therapy, then monthly. |
| > 33% above pretreatment values at 2 consecutive visits (not attributed to other causes) | 11 | DFX dose should be reduced by 10 mg/kg. |
| Progressive increases beyond the ULN | 0 | DFX should be interrupted, then reinitiated at a lower dose followed by gradual dose escalation if the clinical benefit outweighs the potential risks. |
| Pediatrics, > 33% above pretreatment values and above the age-appropriate ULN at 2 consecutive visits | 11 | DFX dose should be reduced by 10 mg/kg. |
| Changes in liver function (elevation in transaminases) | 2 | Liver function should be monitored monthly. After any severe or persistent elevations in serum transaminase levels, dose modifications should be considered. DFX therapy can be cautiously reintroduced once transaminase levels return to baseline. |
| Auditory and ocular alterations | < 1 | Auditory and ophthalmic function should be tested before initiating therapy and annually thereafter. |
| Adverse events . | Incidence in core trials, % . | Monitoring and management . |
|---|---|---|
| DFP-related | ||
| Gastrointestinal (nausea, vomiting, and abdominal pain) | 33 in first y | Mild: continue drug or reduce dose. If severe, discontinue drug temporarily and restart at lower dose. Try liquid formation. 5% of patients discontinue drug permanently. |
| Neutropenia (neutrophils < 1.5 × 109/L) | 8.5 | Monitor blood count weekly for first year, fortnightly in second and subsequent years. Stop drugs for a few weeks. Rechallenge and continue drug if neutrophils at safe level of > 1.5 × 109/L. |
| Agranulocytosis (neutrophils < 0.5 × 109/L) | 1 | Stop drug, treat with intravenous antibiotics if febrile. Give G-CSF if neutropenia prolonged and/or febrile. |
| Rise in transaminases | 7 | Continue to monitor without stopping drug. Enzymes usually fall to normal. If persistently raised > 2 times ULN ( ∼ 1% of patients), discontinue drug. |
| Arthropathy | 3.9-41 | Related to degree of iron overload in some series. Stop drug temporarily and restart at lower dose. ∼ 2% of patients stop drug permanently because of arthropathy. |
| Zinc deficiency | Rare: mainly in diabetes | Give zinc supplements. |
| DFX-related* | ||
| Gastrointestinal | ||
| Diarrhea | 8.8 | Patients should take an antidiarrheal for up to 2 days and keep hydrated. DFX could be taken in the evening rather than the morning. Products, such as Lactaid (if the patient is lactose intolerant) or probiotics (acidophilus or lactobacillus), could be added to the diet. |
| Abdominal pain | 5.0 | Patients should sip water or other clear fluids, and avoid solid food for the first few hours. Avoid narcotic pain medications and nonsteroidal anti-inflammatory drugs. DFX could be taken in the evening rather than the morning. |
| Nausea/vomiting | 14.3 | Patients should drink small, steady amounts of clear liquids, such as electrolyte solutions, and keep hydrated. |
| Skin rash | ||
| Mild to moderate | 4.3 | Likely to resolve spontaneously. DFX should be continued without dose adjustment. |
| Severe | 0.4 | DFX should be interrupted and reintroduced at a lower dose. Patients should take low-dose oral steroids for a short period of time. |
| Renal changes | 36 | Serum creatinine levels should be assessed in duplicate before therapy, then monthly. If patients have additional renal risk factors, serum creatinine levels should be monitored weekly for the first month or after modification of DFX therapy, then monthly. |
| > 33% above pretreatment values at 2 consecutive visits (not attributed to other causes) | 11 | DFX dose should be reduced by 10 mg/kg. |
| Progressive increases beyond the ULN | 0 | DFX should be interrupted, then reinitiated at a lower dose followed by gradual dose escalation if the clinical benefit outweighs the potential risks. |
| Pediatrics, > 33% above pretreatment values and above the age-appropriate ULN at 2 consecutive visits | 11 | DFX dose should be reduced by 10 mg/kg. |
| Changes in liver function (elevation in transaminases) | 2 | Liver function should be monitored monthly. After any severe or persistent elevations in serum transaminase levels, dose modifications should be considered. DFX therapy can be cautiously reintroduced once transaminase levels return to baseline. |
| Auditory and ocular alterations | < 1 | Auditory and ophthalmic function should be tested before initiating therapy and annually thereafter. |
ULN indicates upper limits of normal.
Data from Vichinsky.81
The most serious side effect of DFP is agranulocytosis, a neutrophil count of < 0.5 × 109/L in 2 consecutive blood tests. It occurs in ∼ 1% of patients, most frequently in the first year of treatment, but it has been described in the second year or rarely, later. It is reversible, but some deaths have occurred. The median duration of agranulocytosis is 9 days (range, 3-85 days).
Agranulocytosis has appeared to be most frequent in patients with the Blackfan-Diamond anemia.47,48 The mechanism is unclear. G-CSF may be given. It does not shorten the period of agranulocytosis but speeds recovery once this has begun. Rechallenge with the drug should be avoided; and because of the risk of agranulocytosis, patients receiving DFP should be warned to report immediately any fever or sore throat. We recommend blood tests every week for the first year of therapy and at least every 2 weeks thereafter.
Lesser degrees of neutropenia, neutrophils 0.5-1.5 × 109/L, occur more frequently (Table 3). This is more common in patients with intact spleens and reversible on stopping the drug. Rechallenge is worthwhile because neutropenia may not recur or the neutrophils may settle at a safe, albeit subnormal, level.
An arthropathy affecting mainly large joints, especially the knees, occurs in a proportion of patients. The arthropathy usually resolves after stopping the drug, and often the drug can be successfully reintroduced at the same or a lower dose. Patients may also develop pains in the muscles, which resolve without interrupting therapy.
Transient rises in liver enzymes occur in ∼ 7% of patients, but these usually fall to normal without stopping the drug. In 1% of patients, the rises persist and the drug is then discontinued. The drug does not cause liver fibrosis.33
Zinc deficiency was first reported to occur in diabetic TM patients receiving DFP, and this was associated with increased urine zinc excretion.44 In large trials, there has been a small overall fall in plasma zinc levels but few below the normal range.44 The deficiency is easily detected by measuring serum zinc levels and corrected with zinc supplements without diminishing iron chelation efficacy.
Combined therapy: DFO and DFP
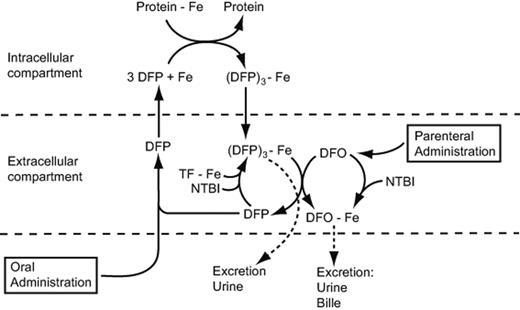
DFP given on each day of the week, and subcutaneous DFO infusions given on some or all of these days was introduced in 1998 for patients inadequately chelated by maximum tolerated doses of DFP.34 The effect of the combined drugs on iron excretion has been found on the basis of urine iron excretion and iron balance studies to be additive or even synergistic.34,50 This has been explained as a shuttle mechanism with DFP entering cells and removing iron, which is then passed on to DFO for excretion in urine or feces51 (Figure 2). The DFP may reenter cells and extract more iron. In addition, recent studies show that DFP is capable of rapidly accessing NTBI fractions in plasma and transferring this iron to DFO.52 Shuttling of iron from DFP to DFO also applies to iron removed from transferrin.53
The “shuttle” mechanism by which DFP given orally binds iron from transferrin (TF), NTBI, and intracellular compartments and transfers some of this iron to DFO. The free DFP is then available to bind more iron. Some DFO also enters cells to bind iron directly.
The “shuttle” mechanism by which DFP given orally binds iron from transferrin (TF), NTBI, and intracellular compartments and transfers some of this iron to DFO. The free DFP is then available to bind more iron. Some DFO also enters cells to bind iron directly.
Combination protocols have differed widely with doses of DFP ranging from 50 to 100 mg/kg and DFO doses from 20 to 60 mg/kg given in addition from 1 to 7 days each week.54 For patients in cardiac failure, DFP is given daily with DFO continuously (Table 4).
Chelation strategies in adult patients with β-TM
| . | DFO* . | DFP . | DFO + DFP combination . | DFX . |
|---|---|---|---|---|
| T2* ≥ 20 ms | ||||
| Iron intake < 0.3 mg/kg/d | ||||
| LIC ≥ 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 7- < 15 mg Fe/g dw | 30-40 mg/kg per day, 8-10 h/d, 5 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/1-2 d + DFP 75 mg/kg/d | 20-30 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 30-40 mg/kg per day, 8-10 h/d, 5 d/wk, SQ | 75 mg/kg/d | Suspend DFO/DFP 75 mg/kg/d | 20-30 mg/kg/d |
| LIC < 3 mg Fe/g dw | Suspend | Suspend | Suspend DFO/Suspend DFP | Suspend |
| Iron intake 0.3-0.5 mg/kg/d | ||||
| LIC ≥ 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 7- < 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/1-2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 30-40 mg/kg per day, 8-10 h/d, 5 d/wk, SQ | 75 mg/kg/d | Suspend DFO/DFP 75 mg/kg/d | 20-30 mg/kg/d |
| LIC < 3 mg Fe/g dw | Suspend | Suspend | Suspend DFO/Suspend DFP | Suspend |
| Iron intake > 0.5 mg/kg/d | ||||
| LIC ≥ 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 7- < 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 30-40 mg/kg per day, 8-10 h/d, 5 d/wk, SQ | 75 mg/kg/d | DFO 40 mg/kg/10-12 h/1 d + DFP 75 mg/kg/d | 20-30 mg/kg/d |
| LIC < 3 mg Fe/g dw | Suspend | Suspend | Suspend DFO/Suspend DFP | Suspend |
| T2* 10- < 20 ms | ||||
| LIC ≥ 15 mg Fe/g dw | 50-60 mg/kg per day, continuous IV | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/7 d + DFP 75 mg/kg/d | 40 mg/kg/d |
| LIC 7- < 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/5 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75-100 mg/kg/d | 30-40 mg/kg/d |
| LIC < 3 mg Fe/g dw | Adjust to therapeutic index,† monitory safety closely | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/1-2 d + DFP 75-100 mg/kg/d/Monitor safety closely | Adjust dose, monitor safety closely |
| T2* < 10 ms | ||||
| LIC ≥ 15 mg Fe/g dw | 50-60 mg/kg per day, continuous IV | Not recommended | DFO 40 mg/kg/10-12 h/7 d + DFP 75-100 mg/kg/d | Not recommended |
| LIC 7- < 15 mg Fe/g dw | 40-50 mg/kg per day, continuous IV | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/5-7 d + DFP 75-100 mg/kg/d | 30-40 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 40-50 mg/kg per day, continuous IV | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/3-5 d + DFP 75-100 mg/kg/d | 30-40 mg/kg/d |
| LIC < 3 mg Fe/g dw | Adjust to therapeutic index,† monitory safety closely | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/1-2 d + DFP 75-100 mg/kg/d | Adjust dose, monitor safety closely |
| . | DFO* . | DFP . | DFO + DFP combination . | DFX . |
|---|---|---|---|---|
| T2* ≥ 20 ms | ||||
| Iron intake < 0.3 mg/kg/d | ||||
| LIC ≥ 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 7- < 15 mg Fe/g dw | 30-40 mg/kg per day, 8-10 h/d, 5 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/1-2 d + DFP 75 mg/kg/d | 20-30 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 30-40 mg/kg per day, 8-10 h/d, 5 d/wk, SQ | 75 mg/kg/d | Suspend DFO/DFP 75 mg/kg/d | 20-30 mg/kg/d |
| LIC < 3 mg Fe/g dw | Suspend | Suspend | Suspend DFO/Suspend DFP | Suspend |
| Iron intake 0.3-0.5 mg/kg/d | ||||
| LIC ≥ 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 7- < 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/1-2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 30-40 mg/kg per day, 8-10 h/d, 5 d/wk, SQ | 75 mg/kg/d | Suspend DFO/DFP 75 mg/kg/d | 20-30 mg/kg/d |
| LIC < 3 mg Fe/g dw | Suspend | Suspend | Suspend DFO/Suspend DFP | Suspend |
| Iron intake > 0.5 mg/kg/d | ||||
| LIC ≥ 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 7- < 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 30-40 mg/kg per day, 8-10 h/d, 5 d/wk, SQ | 75 mg/kg/d | DFO 40 mg/kg/10-12 h/1 d + DFP 75 mg/kg/d | 20-30 mg/kg/d |
| LIC < 3 mg Fe/g dw | Suspend | Suspend | Suspend DFO/Suspend DFP | Suspend |
| T2* 10- < 20 ms | ||||
| LIC ≥ 15 mg Fe/g dw | 50-60 mg/kg per day, continuous IV | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/7 d + DFP 75 mg/kg/d | 40 mg/kg/d |
| LIC 7- < 15 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/5 d + DFP 75 mg/kg/d | 30-40 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 40-50 mg/kg per day, 8-10 h/d, 6 or 7 d/wk, SQ | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/2 d + DFP 75-100 mg/kg/d | 30-40 mg/kg/d |
| LIC < 3 mg Fe/g dw | Adjust to therapeutic index,† monitory safety closely | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/1-2 d + DFP 75-100 mg/kg/d/Monitor safety closely | Adjust dose, monitor safety closely |
| T2* < 10 ms | ||||
| LIC ≥ 15 mg Fe/g dw | 50-60 mg/kg per day, continuous IV | Not recommended | DFO 40 mg/kg/10-12 h/7 d + DFP 75-100 mg/kg/d | Not recommended |
| LIC 7- < 15 mg Fe/g dw | 40-50 mg/kg per day, continuous IV | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/5-7 d + DFP 75-100 mg/kg/d | 30-40 mg/kg/d |
| LIC 3- < 7 mg Fe/g dw | 40-50 mg/kg per day, continuous IV | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/3-5 d + DFP 75-100 mg/kg/d | 30-40 mg/kg/d |
| LIC < 3 mg Fe/g dw | Adjust to therapeutic index,† monitory safety closely | 75-100 mg/kg/d | DFO 40 mg/kg/10-12 h/1-2 d + DFP 75-100 mg/kg/d | Adjust dose, monitor safety closely |
Modified from Brittenham3 with permission.
dw indicates dry weight; SQ, subcutaneous; and IV, intravenous.
Vitamin C-dose limited to 200 mg/day given orally at the time of infusion.
Therapeutic index = mean daily dose (mg/kg; mean daily dose = actual dose of each infusion × doses/7 days)/ferritin (mg/L). Keep index < 0.025 at all times.
Combined chelation can be intensified or reduced by changing the dose of either drug or by varying the number of days each week DFO is infused. Patients comply better with self-administered DFO when this is only needed on 1 or 2 days each week. In addition, the dose of both drugs may be adjusted sufficiently low to avoid side effects of either drug but to still give effective chelation. This has enabled the successful use of combined therapy in children in India.55
Combined therapy with DFO and DFP has been found effective at improving cardiac iron assessed by T2* MRI, LVEF and endothelial function.54,56,57 In Cyprus, where combination therapy with DFP and DFO was introduced for all patients at high risk of heart failure, there was a significant fall in mortality.58 In Italy, a multicenter prospective randomized trial over 7 years in 265 patients found no deaths occurred in patients receiving DFP alone or in combination with DFO, whereas 10 deaths occurred in those receiving DFO alone.59 Lai et al confirmed the superiority of combined therapy over DFO alone in treating established iron-induced cardiac disease.60
In patients who tolerate combined therapy over several years, it is possible to reduce total body iron burden in TM to normal, assessed by serum ferritin and T2* measurement of cardiac and liver iron and to improve endocrine function.61 Improvements in glucose metabolism and gonadal function in both sexes have been achieved.61 This contrasts with single-agent chelator therapy for which there are no reports of significant reversal of endocrine damage. Side effects from the combined therapy have been the same as with either drug alone. There has been no increase in the incidence of agranulocytosis, and no new toxicity.
Alternating therapy: DFP and DFO
The regimen of giving the 2 drugs on different days each week has been termed alternating or sequential therapy. It is aimed at improving compliance with both drugs and at giving some form of chelation every day. In the largest prospective study59,62 in the sequential arm, the patients received DFP 75 mg/kg on 4 days a week and DFO 50 mg/kg on 3 days. Follow-up was a minimum of 5 years. One death from cardiac arrhythmia occurred. In view of the efficacy of and usual compliance with combined DFO and DFP therapy, we have not found it necessary to resort to alternating therapy.
DFX.
DFX is the most recently introduced iron chelator, except in North America where DFX was licensed before DFP. In contrast to DFP, iron excretion is via the fecal route (Table 2). As DFX has a long half-life in plasma, levels are maintained within the therapeutic range over a 24-hour period (Table 2). It can therefore provide 24-hour chelation cover and binding of NTBI with only once daily administration.
To date, DFX clinical experience extends over 9 years, with > 8000 patients investigated across several transfusion-dependent anemias. In a randomized phase 3 trial in 586 patients with TM, a DFX dose of 30 mg/kg per day significantly reduced LIC and serum ferritin. The efficacy of DFX doses of 20 or 30 mg/kg per day was comparable with that of 40-60 mg/kg per day of DFO infused 5 days/wk.63 DFX was also shown to be effective at reducing iron burden in patients who were heavily iron overloaded at baseline64 and who eventually required dose escalation to > 30 mg/kg per day.65 DFX has demonstrated long-term (5 year) dose-dependent efficacy in both adult and pediatric patients66 and was recently shown to be associated with improvement in iron-related hepatic pathology.67 DFX has been associated with greater patient satisfaction and adherence to therapy, and increased time available for normal activities compared with DFO.68
DFX has been found effective in removing iron from the heart in patients with baseline T2* 5-10 ms (severe) and T2* 10-20 ms (mild to moderate iron loading).69-71 Among 71 patients with various degrees of cardiac siderosis, cardiac T2*significantly improved from a mean of 12.0-17.1 ms over a 3-year period.72 LVEF in these patients was normal at the start of the study and did not change. Another study (US04) showed that monotherapy with DFX was effective in chelating cardiac iron in patients with mild to moderate hepatic iron stores but was borderline significant for removal of cardiac iron in patients with severe hepatic iron burden.73
A small number of patients have been treated with twice-daily DFX apparently increasing tolerability and efficacy.74 Until the results of larger studies have been reported, this interesting approach cannot be recommended.
Safety monitoring.
In general, DFX has shown a favorable safety profile at high doses (> 30 mg/kg per day), and in patients achieving serum ferritin levels < 1000 μg/L65,75-81 (Table 3). As for DFP, side effects do not appear more frequent or severe at low iron levels, but we recommend reduction in dose or discontinuation of both drugs when the serum ferritin is < 500 μg/L.
The most common adverse events attributed to DFX therapy are gastrointestinal disturbances and skin rash (Table 3). Diarrhea is more common in the elderly. Mild, nonprogressive increases in serum creatinine and liver enzyme levels have also be noted. Recommendations for their monitoring and management are summarized in Table 3. A boxed warning was added to the United States DFX prescribing information, although this amendment has not been adopted by the European Health Authority or applied globally. The warning indicates that DFX may cause renal and hepatic impairment, including failure, and gastrointestinal hemorrhage. In some reported cases, these reactions were fatal. However, these reactions were observed in patients with advanced age, high-risk MDS, and those with underlying renal or hepatic impairment or low platelet counts. DFX is contraindicated in patients with renal and hepatic failure.81
Combined or alternating therapy: DFX and DFO
As yet, there are no reports of large studies, and we do not recommend these protocols, except in a clinical trial setting. In one study published by abstract only, 15 TM patients with LIC > 15 mg/kg or with lower LIC concentration, but evidence of iron-related organ damage was treated with DFX 20-30 mg/kg daily combined with DFO 35-50 mg/kg subcutaneously on 3-7 days each week.82 Liver iron improved significantly after a mean of 29 weeks. No excessive toxicity was seen. As both DFO and DFX primarily remove liver iron, their combined effects may not be additive as they may compete for the same iron pool. This was so in a gerbil model.83
Sequential therapy of these chelators has been suggested as an attractive option. In a small study of 7 iron-overloaded TM patients, patients received 20-30 mg/kg per day of oral DFX for 4 consecutive days, then a subcutaneous infusion of 20-40 mg/kg per day of DFO for 8-12 hours on the next 3 consecutive days.84 All of the patients showed a decrease in serum ferritin without any side effects. This protocol warrants further evaluation in larger patient numbers, but currently we do not recommend it.
Combined therapy: DFP and DFX
Three studies of combined chelation with the 2 oral chelators DFP and DFX have been reported. In the largest, 16 patients were treated with DFP 75-100 mg/kg per day in 3 divided doses together with DFX 20-25 mg/kg each day.85 There was a fall in total body iron measured by serum ferritin, liver iron and cardiac iron measured by T2* MRI. Improvements occurred in LVEF, gonadal function, and glucose metabolism. Compliance was excellent and quality of life improved for the patients who stopped using DFO infusions. Side effects were no different from those when the drugs are used as monotherapy. In 2 other reports, a total of 4 patients also showed improvement in cardiac iron, cardiac function with excellent compliance, and no unexpected side effects.86,87 Further long term studies in a larger number of patients are needed before this combined, attractive (to patients), oral chelation strategy can be recommended.
FBS0701
FBS0701 is a novel, orally available member of the desazadesferrithiocin class of siderophore-related tridentate chelators currently in clinical development. In preclinical studies, FBS0701 bound Fe(III) with very high affinity and selectivity and demonstrated a > 4-fold higher no-observable-adverse-effect level compared with DFX, suggesting a favorable clinical safety profile, especially with respect to gastrointestinal and renal toxicity.88 Multidose safety and pharmacokinetic studies in iron-overloaded patients established the acute safety of FBS0701 and the feasibility of once-a-day dosing,89 and a phase 2 study has now been reported confirming these observations.90
General principles of iron chelation therapy
Initiating therapy
Before initiation or change of iron chelation therapy, TM patients should be evaluated for the rate of transfusional iron loading (Table 4) and previous chelation. Serum ferritin, LIC, and cardiac T2* MRI and cardiac, hepatic, renal, and endocrine (thyroid, parathyroid, pancreatic, gonadal, and pituitary) function also need to be tested.2,91,92 Potential for pregnancy and the growth and development in children are also assessed.2 The overall prognosis in the chronic anemias other than TM must be assessed. If this is poor (eg, in high-risk myelodysplasia patients), it may not be necessary to institute iron chelation. The same clinical and laboratory tests should be used for initiating and monitoring efficacy of chelation therapy, as for TM.
For patients already satisfactorily chelated on one or other chelator, no change in chelation is needed. Patients in North America and the European Community starting chelation as adolescents or adults have to choose initially between DFO or DFX. After the advantages and disadvantages of the 2 drugs have been explained, most opt for DFX. In some countries (eg, Turkey), DFP is also approved as first-line treatment. It is licensed in the European Community for the treatment of iron overload, in patients with thalassaemia major when deferoxamine therapy is contraindicated or inadequate (Table 2). In the United States, the indication is for the treatment of patients with transfusional iron overload because of thalassaemia syndromes when current chelation therapy is inadequate. Efficacy at tolerable doses, comorbidities, drug side effects, compliance often related to patient preferences, and special patient populations and clinical trials require favoring the use of one regimen over another. These considerations also determine on which chelation regimen the patient is continued. In North America where DFP was only licensed in 2011, DFX is the most widely used, but in the United Kingdom and other parts of the world (eg, India and the Far East) where DFP has been licensed for 10 years or more, DFP alone or in combination with DFO is used by a substantial proportion of patients.90
In TM, we recommend initiating chelation therapy as soon as transfusions have caused enough iron excess to potentially cause tissue damage. Usually in TM, this is at the age of 2 years or older. Current practice is to start after first 10-20 transfusions, when the serum ferritin level is > 1000 μg/L, or when LIC is > 7 mg Fe/g dry weight. Dosing should be tailored according to transfusional iron intake to achieve levels below these thresholds and a cardiac T2* > 20 ms. A discussion with the parents will be needed explaining the advantages and disadvantages of the different drugs.
Maintenance therapy
Maintenance therapy is adjusted to prevent tissue damage because of iron overload.36,91,92 A LIC > 15 mg/g dry weight, serum ferritin > 2500 μg/L or cardiac T2* MRI < 20 ms indicate inadequate chelation. If cardiac iron overload is present (T2* < 20 ms), cardiac iron removal becomes the primary goal of therapy (Table 4). As discussed in the sections dealing with the individual drugs, not all patients are satisfactorily chelated on DFO, DFP, or DFX alone. In many, the dose or frequency of DFO infusions must be revised or the patient switched to another chelator or switched to combined therapy (eg, of DFO with DFP). The iron intake from blood can also be reduced in TM patients by splenectomy if blood requirements are unusually high (> 200-220 mL packed red cells/kg/year).
Monitoring for side effects
This is carried out at appropriate intervals relevant to the chelator or chelators being used (Table 3). Depending on the severity of side effects, reduction of dose, switching to another chelator or use of combined therapy may be needed. In general, side effects with DFO are most frequent at low iron burdens, whereas for DFX and DFP side effects appear to be equivalent at different levels of iron burden.
Monitoring efficacy
Cardiac siderosis.
Intensification of chelation is needed for all patients with a cardiac T2* < 15 ms, whatever the serum ferritin or LIC. Fall in LVEF because of cardiac siderosis or cardiac failure or arrhythmia is best treated by the combination of DFO intravenously (or subcutaneously) at doses of 40-60 mg/kg per day and deferiprone orally 75 mg/kg per day.
Liver iron.
This should be monitored by MRI. Levels > 15 mg/g dry weight indicate that commencement or intensification of chelation is needed. Chelation should be tailored as far as possible to achieve a LIC < 7 mg/g/dry weight.
Special populations
Pediatric patients.
Chelation strategies in the adult TM population (see previous section) can be applied in children with special considerations as follows. Initially in children a dose of DFO 20-30 mg/kg per day is used to avoid toxicity with a maximum dose of 40 mg/kg in children whose growth has ceased.2,91 Close monitoring of growth and bone development is needed if DFO is started at age < 3 years. In the United States (FDA), DFX can also be used to initiate treatment in children as young as 2 years, commencing at a dose of 20 mg/kg. In Europe (European Medicines Evaluation Agency), DFX is only approved as a second-line drug for children younger than 6 years. Compliance in young children may be better to DFO infusions than to oral DFX, but most parents choose DFX. DFX has also had no reported adverse effect on children's growth or on adolescent sexual development in both patients with TM and SCD.66,81 However, monitoring for renal toxicity in children is particularly important.94 A recent study of DFP therapy found that, with the newly introduced liquid formulation, the efficacy and safety profile in 100 children 1-10 years of age was similar to that in older children or adults.46 In developing countries, cost and compliance considerations may make DFP a first choice for children.
Pregnancy.
DFO is the only chelating drug that can be used in pregnancy. It should be interrupted during the first trimester and can be used in the second and third trimesters. A continuous intravenous infusion of DFO (50 mg/kg over 24 hours) can be given before a planned pregnancy.91 DFP and DFX should be stopped in pregnancy and during breast feeding. Sexually active patients receiving DFX or DFP should use contraception.91
Congenital anemias
NTDT.
Chelation in this disease has been discussed at length in a special supplement.95 Nontransfusion-dependent thalassemia (NTDT) describes patients with genetic disorders of hemoglobin synthesis who are not sufficiently severe to warrant regular blood transfusions but are more severely anemic than patients with β- or α-thalassemia trait. Many different genotypes underlie NTDT, β-thalassemia intermedia, hemoglobin E/β thalassemia, hemoglobin H, and hemoglobin E/β thalassemia being the most common.
The patients become progressively iron loaded with increasing age mainly through increased iron absorption. In some patients, transfusions often given at times of infections, during pregnancy or to avoid bone complications, contribute to iron loading. Direct assessment of LIC by biopsy or by MRI is recommended because serum ferritin underestimates iron load in this patient population.96 Chelation is usually started with DFO but switched to one or other oral chelator in those unable or unwilling to comply with DFO.97 In some studies on E/β-thalassemia, iron chelation with DFP has resulted in an improvement in erythropoiesis and hemoglobin levels.98 Clear guidelines are not available, but we use an LIC > 7 mg/g dry weight as an indicator to start iron removal.99 Preliminary data show that DFX is safe and removes iron in TI patients.99,100 A large 1-year randomized, double blind, placebo-controlled phase 2 prospective study on 166 NTDT patients reported DFX to be both safe and efficacious.101 We do not recommend venesections to reduce iron burden because these may aggravate bone abnormalities by increasing anemia.
Blackfan-Diamond anemia.
The indications for commencing iron chelation therapy in Blackfan-Diamond anemia are similar to those in TM. The first report of agranulocytosis with DFP was in an adult patient with Blackfan-Diamond anemia,47 and the drug should be avoided in this condition.48 There have been no unexpected side effects in chelating DBA patients with DFX, and it was effective at lowering LIC and serum ferritin, although less so than in myelodysplasia.102 We recommend to try DFX in patients inadequately chelated on DFO or with hypersensitivity to it.
Aplastic anemia.
The British Society for Hematology Guidelines recommended DFO as first-line chelation therapy for both congenital or acquired aplastic anemia.103 Particular problems may arise because of infections or bleeding at the site of the injection. We recommend DFX as second-line chelator and reserve DFP/DFO for patients with a cardiomyopathy or a T2* < 10 ms. A recent subgroup analysis of 116 patients treated in the EPIC trial with DFX for 1 year found significant reduction in serum ferritin in both chelation naive and previously treated patients. Serum creatinine rose in 25% of the patients, especially in those receiving cyclosporine.78 There were no drug-related cytopenias. A separate study showed that DFX was equally effective as assessed by serum ferritin and labile plasma iron in production or hemolytic anemias.101
Congenital sideroblastic anemia.
The indication for chelation and drugs to be used are similar to those in TM.
SCD.
Blood transfusions have been used in SCD for patients at risk of cerebrovascular accidents or with frequent life-threatening crises. An increasing range of indications have now been identified so that many patients with SCD have received multiple transfusions by adulthood.104
All national guidelines recommend iron chelation in chronically transfused patients with SCD, mainly to avoid liver damage. This is supported by an 11.3% incidence of cirrhosis because of raised total iron burden in an analysis of 141 adult SCD patients over a 25-year review.105
Assessment of SCD-specific populations has demonstrated that elevated iron levels are associated with an increased frequency of acute events, hospitalization, and death. Prospective trials are needed to determine whether the increased iron levels are simply an indicator of the more severely affected patients or increase susceptibility to these other complications. Some studies have shown that patients with SCD with high serum ferritin levels and with a similar number of transfusions to those in TM had normal cardiac T2* values5,106,107 and less endocrine damage.108
The indications for iron chelation therapy in patients with SCD are, however, similar to those as outlined for adults with TM (Table 4). DFO remains the most widely used drug, but compliance with it is particularly poor in SCD patients. A recent study on long-term safety and efficacy of DFX for up to 5 years showed a clinically acceptable safety profile, including maintenance of normal renal function with appropriate DFX dosing, and iron burden was substantially reduced.77 Serial measurement of the glomerular filtration rate and of serum creatinine is indicated during DFX safety monitoring.81,94 Oral therapy with DFP may be preferred if renal damage is present. A recent review of 14 trials found that, among 502 patients, treatment with DFO alone (subcutaneously or intravenously), DFP alone, DFX alone, or combined treatment with DFO and DFP had been used. Only 2 randomized trials had been reported. The authors concluded that the use of chelation in SCD has been based on little efficacy or safety evidence and the cost:benefit ratio had not been fully explored.109 Further prospective studies are clearly needed.
Acquired anemias
Aplastic anemia has already been discussed. Iron-mediated organ damage may occur in multiply transfused, low-risk MDS patients with several reports highlighting that mortality rate is greater in heavily iron-overloaded MDS patients developing hepatic and cardiac dysfunction.110-112 These and other studies have shown an association between high iron levels and increased mortality in MDS treated conventionally or after stem cell transplantation.113,114 It is difficult to be certain, however, how far the iron loading in all these studies directly reduces survival or is a marker for those patients with a poor prognosis because of the length and severity of the MDS.111,115-117 An early T2* MRI study in 11 patients showed that cardiac function and MRI T2* tended to remain normal for a long latent period in MDS patients, even with hepatic iron loading.118 A more recent study in 43 multiply transfused MDS patients found 16.8% with a cardiac T2* < 20 ms.119
Leitch has critically reviewed published data on the benefits and risks of iron chelation therapy in MDS.117 Some studies, often retrospective, have shown improved survival or reduced transformation to acute myeloid leukemia.117,120 A recent matched-pair analysis of 188 iron-loaded MDS patients in which 94 patients received long-term chelation with DFO or DFX and 94 did not, found median survival significantly longer in the chelated group.121 These data support the hypothesis that iron overload plays a role in decreasing survival in multiply transfused low-risk MDS. Emerging data also suggest that iron chelation may be beneficial for overall survival in multiply transfused high-risk MDS and in those selected for stem cell transplantation.
Several studies have shown improvement in white cell and platelets in MDS treated with DFO or DFX.122,123 An improved hemoglobin level, in some cases obviating the need for transfusions has also been described in MDS patients treated with DFO or DFX.117,122,124-126 This effect may be at least partly because of removal of excess iron from the iron- and oxygen-dependent prolyl hydroxylase in the renal oxygen sensing system for erythropoietin production.127,128 Reduction of oxidative stress, which may inhibit hematopoiesis, has also been suggested.123 Prospective randomized trials currently in progress will help to determine which patients with MDS will benefit from chelation therapy whether for leukemic transformation, overall survival, for hepatic or endocrine complications, for transfusion requirements, and for other hematologic parameters.
We recommend that transfusion-dependent MDS patients with an otherwise good prognosis (life expectancy > 1 year) in whom chelation therapy is considered necessary should generally be managed as outlined for TM (Table 4). Various national and international guidelines have been published recommending staring chelation in low and intermediate-1 risk MDS (defined by an international prognostic score) after 20-30 units of blood have been transfused and with serum ferritin levels > 1000 μg/L in some, > 2500 μg/L in other guidelines. Liver and cardiac iron concentrations are also useful in deciding whether or not to start chelation therapy.
Choice of chelation for patients with MDS, chronic myelofibrosis, red cell aplasia, paroxysmal hemoglobinuria, and other severe acquired anemias may not be easy. DFO and DFX are licensed for first-line therapy. DFO may be difficult to administer because of excessive bruising or infection at the infusion site because of cytopenias. On the other hand, extra caution should be used in treating elderly MDS patients or elderly patients with myelofibrosis or other refractory anemias with DFX because of their greater frequency of decreased hepatic, renal, or cardiac function, not related to iron overload, and of concomitant disease or other drug therapy.115-117 DFP is not advisable because of the risk of agranulocytosis, but the incidence of this in MDS is probably no higher than in TM.33 The relative costs of the drugs may influence choice in many countries (Table 2).
Future prospects
The outlook for patients with TM and other transfusion-dependent anemias has improved substantially with the availability of 3 iron-chelating drugs and the use of T2* MRI to detect cardiac siderosis before cardiac symptoms develop. Combined therapy with DFO and DFP has proved particularly effective at treating a previously fatal iron-induced cardiomyopathy. In the United Kingdom, infection rather than iron-induced cardiomyopathy is now the main cause of mortality in TM.23 It seems likely that patient preference and compliance will result in the increased use of the oral chelators and corresponding reduced use of subcutaneous DFO. Randomized trials of oral chelators against DFO may become more difficult to perform because of patient preference. This will be particularly so if a third orally active iron chelator becomes clinically available. With each drug alone, however, a proportion of patients, perhaps 20%, will be inadequately chelated because of lack of efficacy or because the drug dosage has to be reduced or stopped because of side effects. Switching chelators and combination therapy of the oral chelators is likely to increase in use so a randomized trial of the 2 oral chelators DFP and DFX in combination against alternative chelation regimens is urgently needed.
For developing countries, the oral chelators are particularly attractive if the costs can be kept low. Clinical trials are taking place of DFP, which alone of the 3 chelators can cross the blood-brain barrier,129 in conditions, such as Friedrich ataxia and Parkinson disease, with excess iron deposits in the brain. Treatment of diseases, where iron overload is localized to a single organ, will need to be the subject of a future How I Treat review.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professor Dudley Pennell for helpful comments on the sections of the manuscript dealing with measurement of cardiac and liver iron by MRI.
Authorship
Contribution: A.V.H., A.T., and M.D.C. reviewed the relevant literature and wrote the manuscript.
Conflict-of-interest disclosure: A.T. received research funding from Novartis and is a member of the Novartis Speakers Bureau. M.D.C. is a member of the Novartis Speaker Bureau, a member of the Genzyme Advisory Board, and a member of the Shire Advisory Board. A.V.H. declares no competing financial interests.
Correspondence: A. Victor Hoffbrand, Department of Haematology, Royal Free Hospital, Pond Street, London, United Kingdom, NW3 2QG; e-mail: vhoffbrand@googlemail.com.