Abstract
Cytokines within the tumor microenvironment play an important role in supporting the growth and survival of B-cell malignancies. One such cytokine, IL-21, promotes the growth of myeloma and Hodgkin lymphoma cells while inducing apoptosis in chronic lymphocytic leukemia. However, the biologic significance of IL-21 has not been examined in Waldenstrom macroglobulinemia (WM), a B-cell lymphoma characterized by elevated serum IgM and a lymphoplasmacytic bone marrow infiltrate. We report here on the presence of IL-21 in the bone marrow of patients with WM and have identified activated T cells as the source of this cytokine. We readily detected the IL-21 receptor on malignant WM B cells and show that IL-21 significantly increases both IgM secretion and cellular proliferation of these cells with no effect on viability. IL-21 rapidly induces phosphorylation of STAT3 in WM cells, and treatment of the WM cell line MWCL-1 with a STAT3 inhibitor abolished the IL-21–mediated increases in cellular proliferation and IgM secretion. IL-21 also increased the expression of known STAT3 targets involved in B-cell differentiation, including BLIMP-1, XBP-1, IL-6, and IL-10. Overall, our data indicate that IL-21 in the bone marrow microenvironment significantly affects the biology of WM tumor cells through a STAT3-dependent mechanism.
Introduction
Waldenstrom macroglobulinemia (WM), a rare B-cell lymphoma characterized by the presence of a lymphoplasmacytic infiltrate in the bone marrow and high serum levels of monoclonal immunoglobulin M protein (IgM), remains incurable despite significant advances in therapy.1 The lack of a common underlying genetic event leading to the development of WM highlights the potential influence of the bone marrow microenvironment on the pathophysiology of this lymphoma. As with many hematologic malignancies, cytokines in the tumor microenvironment are known to support the growth and proliferation of malignant WM cells and may serve as potential therapeutic targets.2-4 Even more, our recent analysis found a unique cytokine profile in the bone marrow of patients with WM compared with healthy marrows and identified a novel interplay between IL-6, the chemokine RANTES, and IgM production in WM tumor cells.5 These data suggest that other cytokines present within the bone marrow microenvironment may influence the biology of malignant WM cells as well.
Interleukin 21 (IL-21) is the most recently discovered member of the class I cytokine family, which also includes IL-2, IL-4, IL-7, IL-9, and IL-15.6 Like these other cytokines, IL-21 uses a receptor complex to initiate intracellular signaling events. This complex is composed of the common γ chain receptor as well as the specific IL-21 receptor (IL-21R), which is required for ligand binding.7 Although IL-21 is predominantly secreted by activated CD4+ T cell, natural killer T cell, and dendritic cells, IL-21R is expressed by a wide range of effector cells across different lineages, including nearly all cells of the immune system.8,9 However, IL-21 is a pleiotropic cytokine, and its exact biologic effect appears to depend on various factors, including the species and type of effector cell as well as the cell's stage of differentiation and activation status.10,11
In healthy human B cells, IL-21 induces differentiation of CD40- and BCR-activated naive and postswitch memory B cells into antibody-producing plasma cells.12,13 In malignant B cells, however, IL-21 exerts diverse effects, depending on the histology of the malignant cell in question. For example, malignant B cells derived from patients with chronic lymphocytic leukemia respond to IL-21 stimulation by undergoing Bim-dependent apoptosis, an effect that is further enhanced in the presence of chemotherapy and immunotherapy.14,15 Similar proapoptotic effects of IL-21 have been observed in diffuse large B-cell lymphoma and follicular lymphoma as well.16,17 Yet the addition of IL-21 to multiple myeloma and Hodgkin lymphoma cells significantly increased proliferation and prosurvival signals.18-20
These data all indicate a potent, if not differential, effect of IL-21 on the growth and survival of human B cells. However, the biologic significance of IL-21 has not been explored in WM. In the present study we examined the expression of the IL-21 receptor complex on WM cells and have determined whether IL-21 contributes to the biology of this malignancy. Our data show that not only is IL-21 present in the bone marrow microenvironment of patients with WM, but that IL-21 significantly increases the proliferation and IgM secretion of WM tumor cells through a STAT3-mediated mechanism.
Methods
Cell lines and reagents
The WM cell line, MWCL-1, was established by our laboratory and has been characterized previously.21 CD19+CD138+ cells were isolated from the bone marrow of consenting patients with a diagnosis of WM with the use of positive selection beads and RoboSep (StemCell Technologies; Table 1). CD19+CD138+ cells and the remaining CD19−CD138− fraction were cultured separately or frozen in RNALater (Ambion) and stored at −80°C until use in RT-PCR experiments. All cells were cultured in RPMI 1640 (Invitrogen) supplemented with 50 U/mL penicillin G, 10 μg/mL streptomycin, 10% heat-inactivated fetal calf serum, and 1mM sodium pyruvate (Invitrogen) at 37°C with 5% CO2. Recombinant human IL-6 and IL-21 were obtained from PeproTech. The STAT3 inhibitor, STATTIC, was purchased from Tocris Bioscience.
Immunohistochemistry
Paraffin-embedded bone marrow specimens obtained from consenting patients with WM were deparaffinized through xylene and graded alcohols and incubated in a solution of methanol/hydrogen peroxide to quench any endogenous peroxidase activity (Table 1). The sections were then treated with 1mM EDTA (pH = 8) before staining with rabbit anti–human IL-21 (Novus Biologicals) and subsequently with anti–rabbit biotinylated IgG (Dako) and strepdavidin-HRP (Dako). Staining was visualized with 3′,3′-diaminobenzidine and counterstaining with hematoxylin. All slides were observed with light microscopy (Olympus AX70; 200× aperture 0.46, 400× aperture 0.75, 600× aperture 0.80; Olympus America) with images being captured with a SPOT RT camera and software (Diagnostic Instruments). Images were prepared with Adobe Photoshop (Adobe Systems Incorporated).
A novel approach termed SIMPLE (Sequential Immunoperoxidase Labeling and Erasing) method with 3-amino-9-ethylcarbazole (AEC) was used to stain the sections for IL-21 together with either CD3 or CD20.22 The sections were first stained for IL-21, which was detected with Dako Advance System and AEC. The tissue was then destained in 95% alcohol to remove AEC, and the antibody was stripped with glycine/SDS (pH 2) at 50°C for 30 minutes and was rinsed well in TBS. The sections were then stained with either CD3 or CD20 with the use of the same detection system. Digital snapshots of marrow sections were obtained for each antibody before destaining and stripping as described in the previous paragraph. Multicolor composite images were made from subregions of each section and overlaid as separate layers with the use of Adobe Photoshop CS2 with each color assigned to an individual stain.
RT-PCR
Total RNA was isolated with the mirVANA miRNA Isolation Kit (Invitrogen). mRNA (0.5 μg) was reversed transcribed with Superscript III Reverse Transcriptase (Invitrogen), and DNA was amplified with HotStarTaq polymerase (Qiagen) over 35 cycles in a Gene AMP PCR System 9700 thermocycler (Applied Biosystems): 95°C (30 seconds), 55°C annealing (30 seconds), and 72°C extension (30 seconds). Primer sequences were as follows: IL-21 forward, 5′-GAGGAAACCACCTTCCACAA-3′; IL-21 reverse, 5′-CAGGAATCTTCATTCCGTGT-3′; IL-21R forward, 5′-TGCCAACAGGAAGCGAAAGG-3′; IL-21R reverse, 5′-CTCTGGGCTGCGTACCGTACA-3′; GAPDH forward, 5′-GAAGGTGAAGGTCGGAGTC-3′; and GAPDH reverse, 5′-GAAGATGGTGATGGGATTTC-3′. PCR products were then analyzed by 1% agarose gel electrophoresis.
Quantitative RT-PCR (qRT-PCR) was performed in triplicate with the use of EVA Green (Biotium) and HotStarTaq polymerase on a CFX96 Real-time System (Bio-Rad). Primer sequences were as follows: cMYC forward, 5′-CGTCTCCACACATCAGCACAA-3′; cMYC reverse, 5′-TCTTGGCAGCAGGATAGTCCTT-3′; PAX5 forward, 5′-TCCGGAAGCAGATGCGGGGA-3′; PAX5 reverse, 5′-GGGGGACGGTCTCATGGGCT-3′; BIM forward, 5′-TGCCAGCCCTGGCCCTTTTG-3′; and BIM reverse, 5′-CCGCGGTGCTGGGTCTTGTT-3′. Sequences for Bcl-6, BLIMP-1, and XBP-1 have been published previously.23,24
Flow cytometry
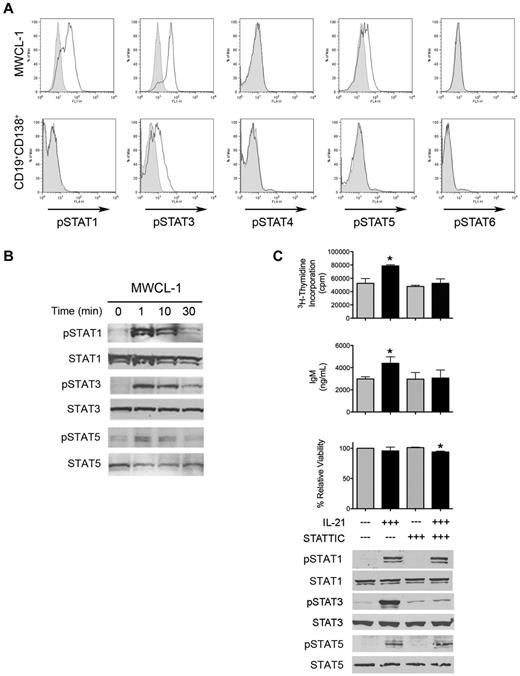
To detect IL-21R and the common γ chain receptor, cells were washed and stained with IL-21R-PE (R&D Biosystems) or common γ chain receptor-APC (Biolegend) antibodies. To determine intracellular IL-21 expression by activated CD4- and CD8-expressing cells, 2 × 106 CD19−CD138− cells isolated from the bone marrow of a patient with WM were cultured on plates coated with anti-CD3 (eBioscience) in the presence of 1 μg/mL soluble CD28 antibody (BD Bioscience). After 96 hours, cells were stimulated with phorbol myristate acetate (10 ng/mL), ionomycin (0.5 μg/mL), and brefeldin A for 4 hours before permeabilization, fixation, and staining with anti–IL-21-PE (eBioscience), anti–CD4-APC, or anti–CD8-FITC (both obtained from BD Biosciences). STAT phosphorylation was assessed in serum-starved MWCL-1 cells and freshly sorted CD19+CD138+ primary tumor cells stimulated with 100 ng/mL IL-21 for 10 minutes. Cells were fixed for 10 minutes at 37°C (BD Phosflow Fix Buffer I; BD Biosciences) and permeabilized for 20 minutes on ice (BD Phosflow Perm Buffer III) according to the manufacturer's directions. Cells were then stained with Alexa Fluor 488– or Alexa Fluor 647–conjugated STAT antibodies (STATs 1-6) or isotype controls. Stained cells were analyzed on a FACSCalibur, and data were processed with FlowJo software (TreeStar Inc). All pSTAT antibodies were purchased from BD Biosciences.
ELISA
Cell-free supernatants were collected from cell cultures and assayed for IL-6, IL-10, IL-21, or IgM according to the respective manufacturers' protocols (Human IL-6, IL-10, and IL-21 ELISA Kits; PeproTech; Human IgM ELISA Kit; Bethyl Laboratories Inc). Optical densities were determined with a SpectraMax190 microplate reader (Molecular Devices).
Proliferation assay
MWCL-1 cells were serum-starved overnight in RPMI containing 1% BSA and cultured for 72 hours with IL-21 in 96-well flat-bottom plates at a density of 0.25 × 105 cells/well. Primary CD19+CD138+ cells were cultured in the presence or absence of IL-21 immediately after sorting at a density of 1 × 105 cells/well of a 96-well plate.
Cultures were pulsed with 1 μCi (0.037 MBq) titrated thymidine (3H-TdR; 5.0 Ci/mmol [185 GBq/mmol]; Amersham) for 18 hours before harvesting, and incorporation of 3H-TdR was determined with a Beckman scintillation counter (GMI Inc).
Annexin-propidium iodide survival staining
Cells cultured in the presence or absence of IL-21 for 72 hours were stained with 1 μg of annexin-V–FITC (Invitrogen) at 4°C for 20 minutes, followed by staining with 0.5 μg of propidium iodide. Cells were immediately analyzed on a FACSCalibur (BD Biosciences). Data analysis was performed with FlowJo Version 6.3.4 software (TreeStar).
Immunoblotting
To determine the kinetics of STAT phosphorylation, MWCL-1 cells were serum-starved overnight in RPMI containing 1% BSA and treated with recombinant IL-21 (100 ng/mL) the next morning. At the indicated times, cells were lysed in RIPA buffer and analyzed by SDS-PAGE. Membranes were stained with pSTAT1, STAT1, pSTAT3, STAT3, pSTAT5, and STAT5. All antibodies were obtained from Cell Signaling except pSTAT3 and STAT3, which were purchased from Santa Cruz Biotechnology. To assess the effects of IL-21 on the expression of various transcription factors, serum-starved MWCL-1 cells were cultured with IL-21 (0-200 ng/mL) for 72 hours. Cells were then lysed with RIPA buffer and analyzed by SDS-PAGE. Membranes were stained with BLIMP-1 (Novus Biologicals), PAX5, Actin, XBP-1 (Santa Cruz Biotechnology Inc), BIM, c-myc, CyclinD1 (Cell Signaling), or Bcl-6 (Abcam).
Statistical analysis
Statistical analysis was performed with Prism Version 5.0d (GraphPad Software) with the use of the Student t test. Significance was set at P < .05.
Results
IL-21 in the WM microenvironment
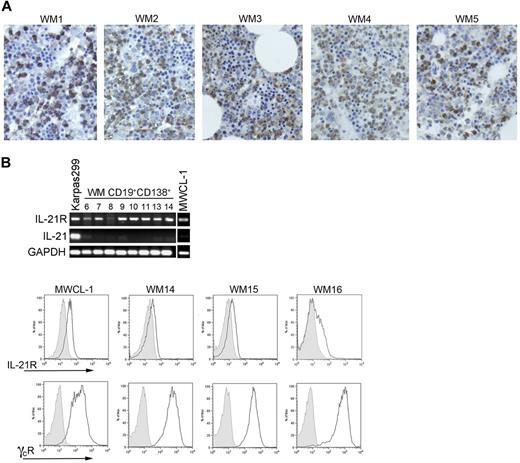
Immunohistochemical staining showed the presence of IL-21 in the bone marrow of patients with WM (Figure 1A), although the IL-21 staining did not differ significantly from that of normal marrow. However, the WM infiltrates in all of the bone marrow sections studied were negative, suggesting that the source of IL-21 within the bone marrow of patients with WM is not the malignant cells. To determine whether the malignant WM B cells are susceptible to the IL-21 present in the bone marrow microenvironment, expression of IL21R was assessed via RT-PCR both in CD19+CD138+ cells isolated by positive selection from patients with WM and in the newly characterized WM cell line, MWCL-1. All CD19+CD138+ patient samples expressed IL21R as did MWCL-1 cells and Karpas 299 cells, which were used as a positive control (Figure 1B). However, as suggested by the immunohistochemical staining, transcript for IL-21 itself was detectable in neither the CD19+CD138+ samples tested nor in the MWCL-1 cell line. With flow cytometry, coexpression of IL-21R and the common γ chain receptor was detected on the MWCL-1 cell line (Figure 1C). Expression of IL-21R was detected on primary CD19+CD138+ WM cells as well at relatively similar levels on all samples assessed, with ΔMFI (mean fluorescence intensity) values (ΔMFIIL-21R/ΔMFIIsotype) ranging from 1.36 to 2.54 (n = 7). The common γ chain receptor was also highly expressed on all primary CD19+CD138+ WM cells studied.
IL-21, IL-21R, and common γ chain receptor expression on WM cells. (A) Immunohistochemical staining of IL-21 (brown) in bone marrow sections obtained from 5 consenting patients with WM (WM1-WM5) was performed with a polyclonal anti–IL-21 antibody as described in “Methods.” Slides were visualized on an Olympic Provus AX70 light microscope, and images shown are with original magnification of ×400. (B) PCR for IL-21R, IL-21, and GADPH was performed on cDNA generated from either CD19+CD138+ cells obtained from patients with WM (n = 8; nos. 6-14) or the WM cell line, MWCL-1. RNA was extracted from previously frozen WM cells; therefore, the sample numbers are not consistent with those noted in other figures. The T-cell line Karpas299 was used as a positive control for both IL-21 and IL-21R. (C) IL-21R and common γ chain receptor coexpression was determined on CD19+CD138+ cells from patients with WM and the MWCL-1 cell line with the use of FACS analysis. Three representative histograms obtained from a total of 8 patient samples are shown (WM14-WM16). Gray histograms represent the appropriate isotype controls.
IL-21, IL-21R, and common γ chain receptor expression on WM cells. (A) Immunohistochemical staining of IL-21 (brown) in bone marrow sections obtained from 5 consenting patients with WM (WM1-WM5) was performed with a polyclonal anti–IL-21 antibody as described in “Methods.” Slides were visualized on an Olympic Provus AX70 light microscope, and images shown are with original magnification of ×400. (B) PCR for IL-21R, IL-21, and GADPH was performed on cDNA generated from either CD19+CD138+ cells obtained from patients with WM (n = 8; nos. 6-14) or the WM cell line, MWCL-1. RNA was extracted from previously frozen WM cells; therefore, the sample numbers are not consistent with those noted in other figures. The T-cell line Karpas299 was used as a positive control for both IL-21 and IL-21R. (C) IL-21R and common γ chain receptor coexpression was determined on CD19+CD138+ cells from patients with WM and the MWCL-1 cell line with the use of FACS analysis. Three representative histograms obtained from a total of 8 patient samples are shown (WM14-WM16). Gray histograms represent the appropriate isotype controls.
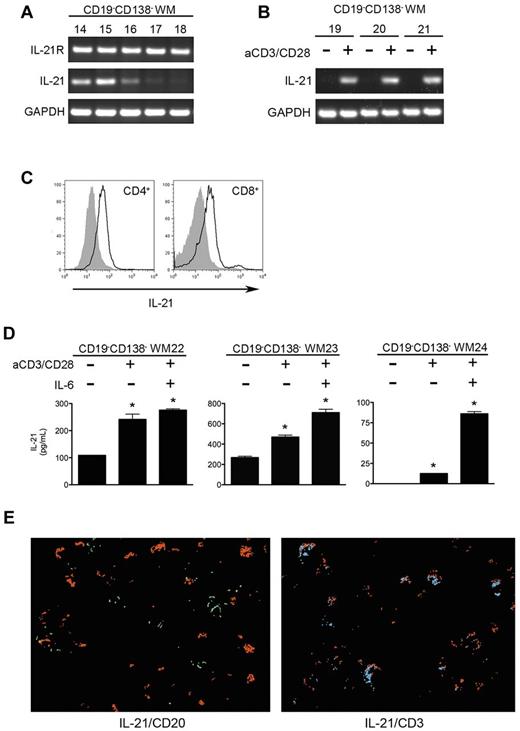
Although WM cells express the receptor complex required for IL-21 binding and intracellular signaling, IL-21 in the WM tumor microenvironment does not appear to come from the malignant B cells. To better understand which cells are expressing IL-21 in the bone marrow of patients with WM, we performed RT-PCR on the CD19−CD138− fraction of cells remaining after positive selection for the malignant B cells (Figure 2A). IL-21 transcript was highly expressed in 3 of the 5 samples tested, with weaker expression in a fourth sample. We cultured additional CD19−CD138− cells obtained from the bone marrow aspirates of patients with WM on plates coated with anti-CD3 and supplemented with anti-CD28 to activate T cells. Cells cultured under these conditions expressed significantly higher levels of IL-21 transcript than did unstimulated cells, suggesting that IL-21 in the WM bone marrow microenvironment is coming from activated T cells (Figure 2B). Furthermore, gating on CD4+ or CD8+ cells within the CD19−CD138− population showed significant expression of intracellular IL-21 protein as well (Figure 2C). We also detected IL-21 expression in activated CD3+CD56+ natural killer T cells, as has been reported previously in mice (data not shown).25 We then detected IL-21 protein in the supernatants of cultured CD19−CD138− cells isolated from WM bone marrow samples (Figure 2D). IL-21 secretion was enhanced when cells were cultured for 72 hours in the presence of anti-CD3 and anti-CD28 as well as IL-6, a cytokine known to stimulate IL-21 production by nonmalignant T cells.26-28 Finally, staining of WM bone marrow sections with IL-21 followed by either CD3 or CD20 showed costaining of IL-21 with CD3+ T cells but not with CD20+ B cells (Figure 2E).
IL-21 in the WM bone marrow microenvironment is derived from activated T cells. (A) PCR for IL-21R, IL-21, and GAPDH was performed on cDNA generated from CD19−CD138− cells obtained from the bone marrows of patients with WM (n = 5; nos. 14-18). (B) Additional CD19−CD138− samples (n = 3; WM6-WM8) were cultured in the presence or absence of anti-CD3 and anti-CD28 for 72 hours to activate T cells. After total RNA extraction, RT-PCR was performed for IL-21 and GAPDH. (C) Flow cytometric analysis for intracellular IL-21 expression in CD19−CD138− WM cells. After culture of CD19−CD138− WM cells on plates coated with anti-CD3 in the presence of 1 μg/mL CD28 antibody for 96 hours, cells were stimulated with phorbol myristate acetate, ionomycin, and brefeldin A as described in “Methods.” To assess IL-21 expression in the activated T-cell population, we gated on the CD4+ and CD8+ populations separately. Shown are histograms representative of 3 different donors. Gray histograms are the respective isotype controls. (D) CD19−CD138− cells obtained from patients with WM (n = 3; WM22-WM24) were cultured for 96 hours in the absence or presence of anti-CD3, soluble CD28 antibody (1 μg/mL), or IL-6 (5 ng/mL) for 96 hours. Cell-free supernatants were collected, and the concentration of IL-21 was assessed by ELISA. All experiments were performed in triplicate, and data are represented as mean ± SD. *Statistically significant at P < .05. (E) Costaining of IL-21 with B cells (CD20) and T-cell (CD3) populations in WM bone marrow. Sections were first stained for IL-21 (red), stripped, and restained for either CD3 (blue) or CD20 (green). Digital images from each layer were then overlaid. A representative multicolor composite image is shown.
IL-21 in the WM bone marrow microenvironment is derived from activated T cells. (A) PCR for IL-21R, IL-21, and GAPDH was performed on cDNA generated from CD19−CD138− cells obtained from the bone marrows of patients with WM (n = 5; nos. 14-18). (B) Additional CD19−CD138− samples (n = 3; WM6-WM8) were cultured in the presence or absence of anti-CD3 and anti-CD28 for 72 hours to activate T cells. After total RNA extraction, RT-PCR was performed for IL-21 and GAPDH. (C) Flow cytometric analysis for intracellular IL-21 expression in CD19−CD138− WM cells. After culture of CD19−CD138− WM cells on plates coated with anti-CD3 in the presence of 1 μg/mL CD28 antibody for 96 hours, cells were stimulated with phorbol myristate acetate, ionomycin, and brefeldin A as described in “Methods.” To assess IL-21 expression in the activated T-cell population, we gated on the CD4+ and CD8+ populations separately. Shown are histograms representative of 3 different donors. Gray histograms are the respective isotype controls. (D) CD19−CD138− cells obtained from patients with WM (n = 3; WM22-WM24) were cultured for 96 hours in the absence or presence of anti-CD3, soluble CD28 antibody (1 μg/mL), or IL-6 (5 ng/mL) for 96 hours. Cell-free supernatants were collected, and the concentration of IL-21 was assessed by ELISA. All experiments were performed in triplicate, and data are represented as mean ± SD. *Statistically significant at P < .05. (E) Costaining of IL-21 with B cells (CD20) and T-cell (CD3) populations in WM bone marrow. Sections were first stained for IL-21 (red), stripped, and restained for either CD3 (blue) or CD20 (green). Digital images from each layer were then overlaid. A representative multicolor composite image is shown.
IL-21 increases cellular proliferation and IgM secretion of malignant WM B cells
We next examined the effect of IL-21 on the biologic activity of malignant WM cells. Unlike previous studies whereby IL-21 was observed to promote apoptosis of malignant B cells, this cytokine had minimal effects on the viability of MWCL-1 cells (Figure 3A). Instead, MWCL-1 cells cultured in IL-21 for 72 hours displayed a dose-dependent increase in cellular proliferation as measured by [3H]-thymidine incorporation (Figure 3B). Similarly, IL-21 significantly enhanced the secretion of IgM by MWCL-1 cells at all concentrations tested (Figure 3C). To confirm that IL-21 was responsible for the increase in IgM secretion by MWCL-1 cells, the fusion protein IL-21R:Fc was used to block IL-21 activity in cocultures of MWCL-1 cells and sorted T cells. Addition of IL-21R:Fc, but not the corresponding IgG control, abolished the enhanced secretion of IgM (Figure 3D).
Biologic effects of IL-21 on the WM cell line, MWCL-1. (A) Viability of MWCL-1 cells cultured in the presence of IL-21 (0-200 ng/mL) for 72 hours. All values are adjusted relative to the viability of the control, which was set to 100%. Proliferation of serum-starved MWCL-1 cells cultured in the presence of IL-21 (0-200 ng/mL). (B) Proliferation was measured at 72 hours by [3H]-thymidine incorporation. (C) IgM secretion by serum-starved MWCL-1 cells cultured in the presence of IL-21 (0-200 ng/mL). After 72 hours of culture, cell-free supernatants were harvested, and IgM levels were determined by ELISA. (D) Sorted T cells (2 × 105) activated with PHA were cultured with 1.5 × 105 MWCL-1 cells for 72 hours in the presence of either hIL-21R:Fc (10 μg/mL) or hIgG (10 μg/mL) and IL-21 (100 ng/mL). IgM secretion was then assessed by ELISA. All experiments were performed in triplicate, and data values represent the mean ± SD of 3 separate experiments. *Statistically significant at P < .05.
Biologic effects of IL-21 on the WM cell line, MWCL-1. (A) Viability of MWCL-1 cells cultured in the presence of IL-21 (0-200 ng/mL) for 72 hours. All values are adjusted relative to the viability of the control, which was set to 100%. Proliferation of serum-starved MWCL-1 cells cultured in the presence of IL-21 (0-200 ng/mL). (B) Proliferation was measured at 72 hours by [3H]-thymidine incorporation. (C) IgM secretion by serum-starved MWCL-1 cells cultured in the presence of IL-21 (0-200 ng/mL). After 72 hours of culture, cell-free supernatants were harvested, and IgM levels were determined by ELISA. (D) Sorted T cells (2 × 105) activated with PHA were cultured with 1.5 × 105 MWCL-1 cells for 72 hours in the presence of either hIL-21R:Fc (10 μg/mL) or hIgG (10 μg/mL) and IL-21 (100 ng/mL). IgM secretion was then assessed by ELISA. All experiments were performed in triplicate, and data values represent the mean ± SD of 3 separate experiments. *Statistically significant at P < .05.
The effects of IL-21 on the MWCL-1 cell line were then confirmed in CD19+CD138+ cells isolated from bone marrow aspirates of patients with WM. Again, IL-21 significantly increased the proliferation of all samples tested (Figure 4A). IL-21 had an even greater effect on inducing IgM secretion by primary WM cells. Although baseline levels of IgM varied widely among samples (an effect that was unrelated to a patient's serum IgM level), addition of IL-21 nearly doubled the level of IgM secretion by cultured cells. (Figure 4B). Similar to what was seen with the MWCL-1 cell line, viability remained relatively unchanged despite 72 hours of culture in the presence of IL-21 (Figure 4C).
Biologic effects of IL-21 on CD19+CD138+ cells obtained from patients with WM (n = 5, WM14, WM15, WM25-WM27). (A) Proliferative effects of IL-21 (25 ng/mL or 100 ng/mL) on CD19+CD138+ cells cultured for 72 hours. Proliferation was assessed by [3H]-thymidine incorporation. Each patient sample was run in quadruplicate, with data presented as the mean ± SD. (B) IgM secretion by CD19+CD138+ cells cultured in the presence or absence of IL-21 (25 ng/mL or 100 ng/mL). After 72 hours, cell-free supernatants were harvested, and IgM levels were determined by ELISA. Samples were run in triplicate, and data are presented as the mean ± SD. The 100-ng/mL well from WM25 became infected and was therefore not usable for this experiment. (C) Viability of CD19+CD138+ cells cultured in the presence or absence of IL-21 (25 ng/mL or 100 ng/mL) for 72 hours. All values are adjusted relative to the viability of the control, which was set at 100%. The 100-ng/mL well from WM25 became infected and was therefore not usable for this experiment. Experiments were performed once for each patient sample. *Statistically significant at P < .05.
Biologic effects of IL-21 on CD19+CD138+ cells obtained from patients with WM (n = 5, WM14, WM15, WM25-WM27). (A) Proliferative effects of IL-21 (25 ng/mL or 100 ng/mL) on CD19+CD138+ cells cultured for 72 hours. Proliferation was assessed by [3H]-thymidine incorporation. Each patient sample was run in quadruplicate, with data presented as the mean ± SD. (B) IgM secretion by CD19+CD138+ cells cultured in the presence or absence of IL-21 (25 ng/mL or 100 ng/mL). After 72 hours, cell-free supernatants were harvested, and IgM levels were determined by ELISA. Samples were run in triplicate, and data are presented as the mean ± SD. The 100-ng/mL well from WM25 became infected and was therefore not usable for this experiment. (C) Viability of CD19+CD138+ cells cultured in the presence or absence of IL-21 (25 ng/mL or 100 ng/mL) for 72 hours. All values are adjusted relative to the viability of the control, which was set at 100%. The 100-ng/mL well from WM25 became infected and was therefore not usable for this experiment. Experiments were performed once for each patient sample. *Statistically significant at P < .05.
IL-21 activates STAT signaling in WM cells
Because IL-21 produced a significant biologic effect on the proliferation and IgM secretion of WM cells, we next determined which signaling mechanisms were responsible for mediating the effects of this cytokine in WM. We specifically explored the phosphorylation status of members of the STAT family of signaling molecules, because the JAK/STAT signaling pathway, in particular STAT3, is known to be activated on the binding of IL-21 to its cognate receptor.14,18,20,28 Stimulation with 100 ng/mL IL-21 for 10 minutes yielded significant increases in the phosphorylation status of STAT1 and STAT3, and, to a lesser extent, STAT5 in serum-starved MWCL-1 cells (Figure 5A). Although STAT activation was more subtle in CD19+CD138+ WM cells obtained from patient bone marrow samples, phosphorylation of STAT3 was still significantly enhanced after incubation with 100 ng/mL IL-21 (Figure 5A). Further analysis was performed to discover the kinetics of STAT activation in response to IL-21 stimulation. Increases in the phosphorylation of STAT1, STAT3, and STAT5 were detectable in MWCL-1 cells within 1 minute after the addition of IL-21 and were sustained to 30 minutes (Figure 5B). We then examined the effect of STAT3 inhibition on the biologic response of MWCL-1 cells to IL-21 through the use of the STAT3-specific small-molecule inhibitor, STATTIC.29 Inhibition of STAT3 phosphorylation by STATTIC abrogated the IL-21–mediated increase in both proliferation and IgM secretion observed in the absence of STATTIC (Figure 5C). Cells cultured in 0.5μM STATTIC were also unable to respond to IL-21 with an increase in STAT3 phosphorylation, whereas STAT1 and STAT5 phosphorylation remained unaffected, providing further evidence for the importance of STAT3 as the primary mediator of IL-21 signaling in WM cells.
IL-21 mediates its biologic effects through activation of STAT3. (A) Serum-starved MWCL-1 or freshly sorted CD19+CD138+ WM tumor cells (n = 3) were stimulated with 100 ng/mL IL-21 for 10 minutes. After fixation and permeabilization, tyrosine phosphorylation of STATs 1, 3, 4, 5, and 6 was determined via FACS analysis. Shown is a representative example of 3 separate experiments. Gray histograms indicate baseline STAT phosphorylation, with open histograms representing expression of the phosphorylated isoform. (B) Immunoblot analysis of tyrosine phosphorylation of STATs 1, 3, and 5 in serum-starved MWCL-1 cells treated with 100 ng/mL IL-21 for the indicated times. Total STAT1, STAT3, and STAT5 were used as loading controls. Shown are representative blots of 3 separate experiments. (C) Inhibition of IL-21 activity through the use of the STAT3 inhibitor, STATTIC. MWCL-1 cells were pretreated for 20 minutes in the presence or absence of 0.5μM STATTIC before the addition of 100 ng/mL IL-21 where indicated. Cells were cultured for 72 hours, and proliferation, IgM secretion, and viability were assessed as outlined in “Methods.” Each experiment was performed 3 times, and data represent the mean ± SD. *Statistically significant at P < .05. Immunoblotting for pSTAT3 was performed on MWCL-1 cells pretreated in the presence or absence of 0.5μM STATTIC for 20 minutes, followed by stimulation with 100 ng/mL IL-21 for 10 minutes. Cells were lysed in RIPA buffer and SDS-PAGE was performed. After probing for pSTAT3, blots were stripped, and total STAT3 was used as a loading control.
IL-21 mediates its biologic effects through activation of STAT3. (A) Serum-starved MWCL-1 or freshly sorted CD19+CD138+ WM tumor cells (n = 3) were stimulated with 100 ng/mL IL-21 for 10 minutes. After fixation and permeabilization, tyrosine phosphorylation of STATs 1, 3, 4, 5, and 6 was determined via FACS analysis. Shown is a representative example of 3 separate experiments. Gray histograms indicate baseline STAT phosphorylation, with open histograms representing expression of the phosphorylated isoform. (B) Immunoblot analysis of tyrosine phosphorylation of STATs 1, 3, and 5 in serum-starved MWCL-1 cells treated with 100 ng/mL IL-21 for the indicated times. Total STAT1, STAT3, and STAT5 were used as loading controls. Shown are representative blots of 3 separate experiments. (C) Inhibition of IL-21 activity through the use of the STAT3 inhibitor, STATTIC. MWCL-1 cells were pretreated for 20 minutes in the presence or absence of 0.5μM STATTIC before the addition of 100 ng/mL IL-21 where indicated. Cells were cultured for 72 hours, and proliferation, IgM secretion, and viability were assessed as outlined in “Methods.” Each experiment was performed 3 times, and data represent the mean ± SD. *Statistically significant at P < .05. Immunoblotting for pSTAT3 was performed on MWCL-1 cells pretreated in the presence or absence of 0.5μM STATTIC for 20 minutes, followed by stimulation with 100 ng/mL IL-21 for 10 minutes. Cells were lysed in RIPA buffer and SDS-PAGE was performed. After probing for pSTAT3, blots were stripped, and total STAT3 was used as a loading control.
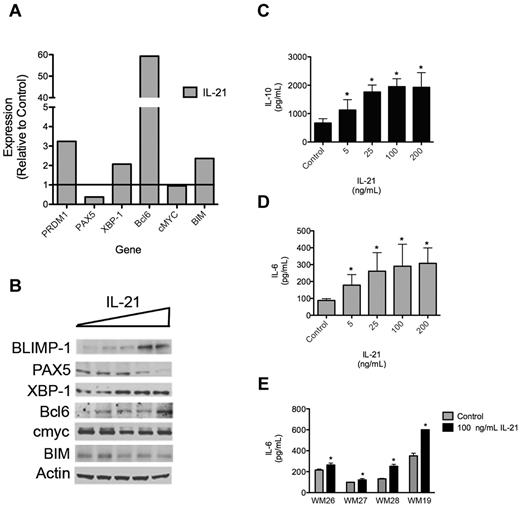
IL-21 regulates the expression of genes involved in B-cell differentiation
To better understand the mechanisms by which IL-21 increases cellular proliferation and IgM secretion in malignant WM B cells, we assessed the effect of IL-21 on the mRNA and protein expression of several transcription factors known to regulate healthy B-cell differentiation. qRT-PCR and Western blot analysis indicated increases in both the transcript and protein expression of BLIMP-1 (PRDM1), XBP-1, and Bcl6 on the addition of IL-21 to the culture media (Figure 6A-B). PAX5, which is suppressed by BLIMP-1, was subsequently decreased at both the mRNA and protein levels.30 Transcript and protein expression of XBP-1, a transcription factor involved in regulating immunoglobulin secretion, was also enhanced in the presence of IL-21, consistent with the increase in IgM secretion observed when WM cells are cultured with IL-21. IL-21 had little effect on cMyc expression in MWCL-1 cells despite this transcription factor being a known target of IL-21.16 However, cMyc expression was already quite high in the MWCL-1 cells, and it is possible that additional stimulation by IL-21 yielded no additional affect. Finally, gene expression of the proapoptotic Bcl-2 family member BIM, which has been shown to have an important role in mediating the effects of IL-21 in chronic lymphocytic leukemia, was increased in MWCL-1 cells treated with IL-21.15 However, this increase in transcript did not translate to an increase in BIM protein level, consistent with the minimal effects of IL-21 on apoptosis in WM.
IL-21 regulates gene and protein expression in WM. (A) qRT-PCR analysis of transcription factors involved in B-cell differentiation. MWCL-1 cells were treated with 100 ng/mL IL-21 for 48 hours, at which time mRNA was extracted and qRT-PCR was performed. Data are presented as the fold change in expression relative to untreated MWCL-1, with a value of 1 indicating no change in transcript expression on treatment with IL-21. Representative data from 1 of 3 separate experiments are shown. (B) Immunoblot analysis of transcription factors involved in B-cell differentiation. MWCL-1 cells were treated with increasing concentrations of IL-21 (0-200 ng/mL) for 72 hours, at which time cells were lysed with RIPA buffer and SDS-PAGE was performed. After probing for specific transcription factors, blots were stripped and reprobed for actin, which was used as a loading control. Shown are representative blots of 3 separate experiments. (C-D) ELISA was performed for (C) IL-10 or (D) IL-6 with the use of cell-free supernatants collected from MWCL-1 cells cultured in IL-21 for 72 hours. Each experiment was performed in triplicate on 3 separate occasions, and data represent the mean ± SD. (E) Freshly sorted CD19+CD138+ WM tumor cells (n = 4; WM26-28, WM19) were cultured with or without IL-21 for 72 hours. Cell-free supernatants were collected, and the concentration of IL-6 was determined by ELISA. Data represent the mean ± SD of a single experiment performed in triplicate. *Statistically significant at P < .05.
IL-21 regulates gene and protein expression in WM. (A) qRT-PCR analysis of transcription factors involved in B-cell differentiation. MWCL-1 cells were treated with 100 ng/mL IL-21 for 48 hours, at which time mRNA was extracted and qRT-PCR was performed. Data are presented as the fold change in expression relative to untreated MWCL-1, with a value of 1 indicating no change in transcript expression on treatment with IL-21. Representative data from 1 of 3 separate experiments are shown. (B) Immunoblot analysis of transcription factors involved in B-cell differentiation. MWCL-1 cells were treated with increasing concentrations of IL-21 (0-200 ng/mL) for 72 hours, at which time cells were lysed with RIPA buffer and SDS-PAGE was performed. After probing for specific transcription factors, blots were stripped and reprobed for actin, which was used as a loading control. Shown are representative blots of 3 separate experiments. (C-D) ELISA was performed for (C) IL-10 or (D) IL-6 with the use of cell-free supernatants collected from MWCL-1 cells cultured in IL-21 for 72 hours. Each experiment was performed in triplicate on 3 separate occasions, and data represent the mean ± SD. (E) Freshly sorted CD19+CD138+ WM tumor cells (n = 4; WM26-28, WM19) were cultured with or without IL-21 for 72 hours. Cell-free supernatants were collected, and the concentration of IL-6 was determined by ELISA. Data represent the mean ± SD of a single experiment performed in triplicate. *Statistically significant at P < .05.
We also determined whether IL-21 affected the expression and secretion of other cytokines known to be involved in the pathophysiology of WM tumors and regulated through STAT3. Specifically, IL-21 was observed to increase the secretion of IL-6 by both MWCL-1 cells and primary CD19+CD138+ tumor cells (Figure 6B-C). Significantly higher levels of IL-10 were also detected in the cell-free supernatants of MWCL-1 cells treated with IL-21 than of control cells. However, use of neither an IL-6R nor IL-10R neutralizing antibody before administration of IL-21 diminished the biologic response of MWCL-1 cells to IL-21, suggesting that these effects are because of IL-21 itself and not a secondary increase in IL-6 or IL-10 (data not shown).
Discussion
IL-21 is known to be an integral mediator of B-cell differentiation and antibody production. IL21R−/IL21R− mice have normal B-cell counts but elevated titers of IgE along with severe defects in IgG production after antigen stimulation, whereas IL-21 transgenic mice exhibit elevated serum levels of IgM and IgG1, indicating the ability of IL-21 to promote isotype switching in vivo.31,32 IL-21 is also a potent stimulator of class-switch recombination and plasma cell differentiation in human B cells.13 Because of its known role in the development of healthy B cells, investigations have been undertaken to explore the effect of IL-21 on malignant B cells. Interestingly, the response to IL-21 in malignancy highly depends on the differentiation status and pathology of the malignant B cell under study.15,17,18,33
Although cytokines within the tumor microenvironment are known to be important in the maintenance of WM tumors, the biologic response of malignant WM B cells to IL-21 has not been examined. We report here that IL-21 is not only present within the bone marrow of patients with WM but also that both the representative WM cell line, MWCL-1, and primary CD19+CD138+ WM cells express both components of the receptor complex required for IL-21 binding and signaling, namely the IL-21R and the common γ chain receptor.8 These findings led us to believe that WM tumor cells may be responsive to the IL-21 in their microenvironment.
To evaluate the biologic significance of IL-21 in the bone marrow of patients with WM, we examined the effect of IL-21 on the proliferation of isolated WM tumor cells. CD19+CD138+ WM cells cultured with IL-21 for 72 hours showed a significant increase in proliferation, an effect that was also observed in MWCL-1 cells. The effect of IL-21 on IgM secretion was even more substantial. However, IL-21 appeared to have little effect on the viability of WM cells. These findings are consistent with previous studies in Hodgkin lymphoma, Burkitt lymphoma, and multiple myeloma whereby IL-21 was observed to promote the growth of malignant B cells.17,18,33 However, although the ability of IL-21 to increase antibody production by healthy B cells has been well characterized, ours is the first study to report an increase in immunoglobulin secretion by malignant B cells after culture with IL-21. Because WM is a lymphoma defined by elevated levels of IgM in the sera, often leading to hyperviscosity and related physical findings, our data suggest that strategies to block IL-21 may be useful in lowering the levels of IgM secreted by these tumors and may serve as novel therapeutic agents in the treatment of this malignancy.
Here, we have identified activated CD4+ and CD8+ T cells as being the primary source of IL-21 in the bone marrows of patients with WM.9,26 Although most of the CD19−CD138− samples obtained from bone marrow aspirates expressed IL-21 transcript at baseline, culture of these cells in the presence of anti-CD3 and anti-CD28 significantly enhanced both the expression and secretion of IL-21. A recent study has identified IL-6 as a specific and potent inducer of IL-21 production by CD4+ T cells, and accordingly we observed significant increases in the secretion of IL-21 into the supernatant of CD19−CD138− cell cultures when IL-6 was added to the culture medium.26 Gene expression analyses have identified IL-6 and its cognate receptor as being 2 of the most highly up-regulated genes in malignant WM cells compared with normal B cells, and recent findings from our laboratory indicate significantly elevated levels of soluble IL-6 in both peripheral and bone marrow sera obtained from patients with WM.5,34 Although IL-6 itself is known to promote B-cell differentiation and antibody secretion from WM cells, our data suggest that IL-6 in the bone marrow may be acting on T cells as well, subsequently enhancing the production and secretion of IL-21 into the tumor microenvironment where it is then free to act on malignant WM cells, increasing their cellular proliferation and IgM secretion.5 Furthermore, we detected a significant increase in the secretion of IL-6 from both the MWCL-1 cell line and CD19+CD138+ WM cells cultured with IL-21. However, culturing WM cells with both IL-21 and IL-6 together had no additional effect on cellular proliferation or IgM secretion compared with IL-21 alone (data not shown). In addition, use of an IL-6R–neutralizing antibody before the addition of IL-21 did not diminish the IL-21–mediated increase in cell growth and antibody production, suggesting that the effects of IL-21 on malignant WM cells are not because of a secondary increase in IL-6. Taken together, these data indicate the potential for a paracrine signaling loop that involves the production of IL-6 by malignant WM B cells and a subsequent increase in IL-21 expression and secretion by neighboring T cells within the tumor microenvironment, which ultimately promotes cellular proliferation and IgM secretion by the tumor cells through the activation of STAT3.
In addition to IL-6, the expression of other STAT3 targets was increased in WM cells treated with IL-21 as well. BLIMP-1, which is considered to be the master regulator of plasma cell differentiation, is encoded by PRDM1, a gene that is directly regulated by STAT3 after treatment of splenic B cells with IL-21.35 In MWCL-1 cells cultured with IL-21, we noted increases in BLIMP-1 mRNA and protein levels as well. Accordingly, expression of PAX5, a target of BLIMP-1 repression, was subsequently decreased, an event that has been shown to be necessary for the differentiation of healthy B cells into antibody-secreting plasma cells.36 Expression of XBP-1, which is the proximal regulator of antibody secretion from plasma cells and is normally suppressed by PAX5, was increased in MWCL-1 cells, consistent with the increase in IgM secretion by WM cells on treatment with IL-21. The changes in the expression of these proteins are all consistent with the differentiation of a B cell toward the secretory phenotype of a plasma cell. Interestingly, no effect of IL-21 was observed on the expression of cMyc protein levels, despite a known regulation of cMyc expression by STAT3.16
In conclusion, we have identified the presence of IL-21 in the bone marrow of patients with WM. Although malignant B cells express the necessary components required for IL-21 binding and signaling, IL-21 cytokine appears to come not from the malignant cells themselves but from activated T cells present in the tumor microenvironment. IL-21 not only increases the proliferation of WM cells but also enhances the secretion of IgM and IL-6 as well, an effect of IL-21 that has not previously been found in malignant B cells. We believe that these data may indicate a novel interplay between malignant B cells and T cells within the bone marrow of patients with WM. Further investigations into the effectiveness of blocking IL-21 or its primary signaling mediator, STAT3, therapeutically for the treatment of WM are warranted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.S.H. developed the experimental design, performed experiments, analyzed data, and prepared the manuscript; S.C.Z. performed immunohistochemical staining and associated data analysis; M.A.G. provided patient samples; Z.Z.Y. and F.J.S. participated in study design and data analysis; A.J.N. and S.M.A. assisted with study design, data analysis, and manuscript preparation. All authors have read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen M. Ansell, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: ansell.stephen@mayo.edu.

![Figure 3. Biologic effects of IL-21 on the WM cell line, MWCL-1. (A) Viability of MWCL-1 cells cultured in the presence of IL-21 (0-200 ng/mL) for 72 hours. All values are adjusted relative to the viability of the control, which was set to 100%. Proliferation of serum-starved MWCL-1 cells cultured in the presence of IL-21 (0-200 ng/mL). (B) Proliferation was measured at 72 hours by [3H]-thymidine incorporation. (C) IgM secretion by serum-starved MWCL-1 cells cultured in the presence of IL-21 (0-200 ng/mL). After 72 hours of culture, cell-free supernatants were harvested, and IgM levels were determined by ELISA. (D) Sorted T cells (2 × 105) activated with PHA were cultured with 1.5 × 105 MWCL-1 cells for 72 hours in the presence of either hIL-21R:Fc (10 μg/mL) or hIgG (10 μg/mL) and IL-21 (100 ng/mL). IgM secretion was then assessed by ELISA. All experiments were performed in triplicate, and data values represent the mean ± SD of 3 separate experiments. *Statistically significant at P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/18/10.1182_blood-2012-03-419440/4/m_zh89991298700003.jpeg?Expires=1769939874&Signature=zxxrpceoT6MalukFrezsKhNljYSSEbcH35o2dcRbLlGjk0jBy34lS7Htbc7fUpX5n0r8K1OTinPmOOkHgWg~lKy6rJNvc92VDvAD1MtZx1hB3MIG4hnQF3CXddBz-X~7PS6ETbbPL8BHou3gHOOKCHqjuCnItnuVXFROypVTJRrlHrM93uU5BAPlD~ueqI1FqBg82brz8ZACPvNcBcHgZS~gAixkBEgOF7-9DM1wDcGTEGg3suxNJyJihFGrV6aEz3tRY0s4qsEtS3AhKHIquI0txdyda9AM0~dG9-NRvt4SQ15bAWFOInXEWYlyo8BtS25AU~o~jU5IDgrKMCbG2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Biologic effects of IL-21 on CD19+CD138+ cells obtained from patients with WM (n = 5, WM14, WM15, WM25-WM27). (A) Proliferative effects of IL-21 (25 ng/mL or 100 ng/mL) on CD19+CD138+ cells cultured for 72 hours. Proliferation was assessed by [3H]-thymidine incorporation. Each patient sample was run in quadruplicate, with data presented as the mean ± SD. (B) IgM secretion by CD19+CD138+ cells cultured in the presence or absence of IL-21 (25 ng/mL or 100 ng/mL). After 72 hours, cell-free supernatants were harvested, and IgM levels were determined by ELISA. Samples were run in triplicate, and data are presented as the mean ± SD. The 100-ng/mL well from WM25 became infected and was therefore not usable for this experiment. (C) Viability of CD19+CD138+ cells cultured in the presence or absence of IL-21 (25 ng/mL or 100 ng/mL) for 72 hours. All values are adjusted relative to the viability of the control, which was set at 100%. The 100-ng/mL well from WM25 became infected and was therefore not usable for this experiment. Experiments were performed once for each patient sample. *Statistically significant at P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/18/10.1182_blood-2012-03-419440/4/m_zh89991298700004.jpeg?Expires=1769939874&Signature=I4NLw682uGe6hpgsyjD~VZC0Mu-uePGZWC0GxFkcfWEXPqU1s-rVw91He-iYeJO1ZTlc8PLGjFE2Jnl~goYCC6uz4fpZhGdtJ~OSLLyGS~crEQsAOlZvb2RHtCJEWObR1eFcrAskfvxp1C4w2rKW77ANBNDHwz7FvYNx0ZSOvOUykdIGELRiakQThCIx6MXxu6UZh4BUFEdPI~YXCoafS6pJfunT93XYKFqLAnCe8gRzzTOzjKDuiNr7vvsPIBm~fYnVNQyK-SFIgmiz~JnUSi2tdcIb7qa6lhGn9OuXYswVxduC0lLvREo2hILPuTB8hldqDg24H3XA3AaQJrevng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)