Abstract
Eosinophils are the major cellular effectors of allergic inflammation and represent an important therapeutic target. Although the genesis and activation of eosinophils have been extensively explored, little is known about their intravascular kinetics or physiological fate. This study was designed to determine the intravascular life span of eosinophils, their partitioning between circulating and marginated pools, and sites of disposal in healthy persons. Using autologous, minimally manipulated 111-Indium–labeled leukocytes with blood sampling, we measured the eosinophil intravascular residence time as 25.2 hours (compared with 10.3 hours for neutrophils) and demonstrated a substantial marginated eosinophil pool. γ camera imaging studies using purified eosinophils demonstrated initial retention in the lungs, with early redistribution to the liver and spleen, and evidence of recirculation from a hepatic pool. This work provides the first in vivo measurements of eosinophil kinetics in healthy volunteers and shows that 111-Indium–labeled eosinophils can be used to monitor the fate of eosinophils noninvasively.
Introduction
Eosinophils play a key role in allergic inflammation1 and represent an important therapeutic target in asthma and other allergic diseases. They have the capacity to release histotoxic substances, including granule proteins, inflammatory cytokines, and reactive oxygen metabolites, which cause bronchoconstriction, epithelial damage, hyper-responsiveness, and airway remodeling.2-6
Much is known about the cellular mechanisms regulating the development and maturation of eosinophils, their release from the bone marrow, and the processes involved in their recruitment, activation, and clearance during allergic inflammation.7-11 By contrast, very little is known about the physiology of circulating eosinophils in humans. Because of the relative scarcity of eosinophils in the blood of healthy persons (range, 0.0-0.4 × 109/L), previous attempts to study eosinophil kinetics have been restricted to patients with hypereosinophilia,12-14 hampered by label reuse after pulse injection of 3H-thymidine,15 or relied on autoradiographs developed > 500 days.16 We have used 111-Indium–labeled mixed leukocytes with postinjection isolation of eosinophils to ascertain their intravascular life span, and subsequently purified 111-Indium–labeled autologous eosinophils with γ camera imaging to assess organ-specific trafficking in vivo. We have demonstrated an intravascular lifespan for circulating eosinophils exceeding 24 hours and revealed extensive intravascular margination of these cells, together with evidence of recirculation from a hepatic pool.
Methods
Participants
Healthy male and female adults with normal lung function and eosinophil counts (range, 0.02-0.38 × 109/L) gave written informed consent in accordance with the Declaration of Helsinki. The study was approved by Cambridgeshire Research Ethics Committee (09/H0308/119) and the Administration of Radioactive Substances Advisory Committee of the United Kingdom (83/3130/25000).
Leukocyte labeling
Mixed leukocytes isolated from blood by hetastarch sedimentation17 were labeled with 111-Indium tropolonate and reinjected into an antecubital vein. Administered activities ranged from 8.3 to 13.0 MBq. Venous blood (40 mL) was sampled at 0.75, 2, 4, 6, 9, 12, 24, 48, and 72 hours after reinjection. From each sample, neutrophils and eosinophils were isolated in parallel to ≥ 99% purity using the RoboSep system (StemCell Technologies), and the radioactivity was measured using a γ counter.
Eosinophil labeling
Eosinophils were isolated from autologous venous blood using plasma-Percoll gradients17 followed by immunomagnetic separation with clinical grade anti-CD16 microbeads (CliniMACS; Miltenyi Biotec). Isolated eosinophils (91% ± 1% pure) were labeled with 111-Indium tropolonate (0.73 ± 0.15 MBq/106 cells).
Eosinophil activation status
The activation status of the 111-Indium–labeled eosinophils was assessed by cell surface marker expression (CD69, CD44, CD81, and CD66b), eosinophil shape change, transmission electron microscopy analysis of cell morphology, and eosinophil-derived neurotoxin granule release (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Analysis
Percentage granulocyte recovery was calculated using the formula:
Neutrophil or eosinophil recovery (%) = neutrophil or eosinophil-associated activity (cpm/mL) × blood volume (mL)/injected neutrophil or eosinophil-associated activity (cpm/mL).18
Blood volume was estimated as described.19 An automated differential leukocyte count was obtained (LH750; Beckman Coulter) and used to correct for the efficiency of the isolation for each individual recovery. Intravascular life span was determined by dividing the area under the time-activity curve by the 45-minute recovery. Values are expressed as mean ± SEM. P < .05 was considered significant.
Dynamic imaging
Volunteers lay supine above a double-headed γ camera (Elscint, Apex SPX Helix) fitted with a medium-energy, parallel-hole collimator. After bolus injection of labeled eosinophils, activities in the chest and abdomen were recorded by imaging with a frame time of 1 second for 2 minutes followed by 20 seconds for 38 minutes. At later time points, there was a single frame time of 10 minutes. To generate organ time-activity curves, regions of interest were drawn over the right and left lungs, right ventricle, bone marrow (pelvis), liver, and spleen using Xeleris Version 3.0423 software (GE Healthcare). Counts per MBq injected in these regions were recorded and corrected for 111-Indium physical decay.
Results and discussion
To determine the kinetics of minimally manipulated eosinophils, mixed leukocytes (supplemental Figure 1) were isolated from peripheral blood, labeled with 111-Indium, and reinjected. These experiments were considered essential, given the potential for ex vivo cell activation to affect the subsequent behavior of cells when reinjected.20 The 45-minute recovery value is indicative of the proportion of labeled cells remaining in the freely circulating blood pool (rather than sequestered in tissue vascular beds). We obtained a 45-minute recovery for neutrophils of 57% ± 10% (SEM; n = 7), which is consistent with previous values20 and thus validates our methodology. This was followed by monoexponential removal from the circulation (Figure 1A). Interestingly, eosinophils isolated in parallel from the same blood samples displayed markedly different kinetics (Figure 1B). First, 45-minute eosinophil recovery (15% ± 2%) was lower than the corresponding value for neutrophils, suggesting a larger pool of marginated eosinophils. Second, after an initial decline in labeled eosinophils between 45 minutes and 2 hours, the number in the circulation recovered at 4 and 9 hours (suggesting possible recirculation) before final monoexponential removal. Third, the intravascular life span of eosinophils was 25.2 ± 3.8 hours compared with 10.3 ± 0.1 hours for neutrophils. Plasma radioactivity was ≤ 2% indicating stable cell association of the 111-Indium label. Although even the minimally manipulated labeled mixed leukocyte population may be susceptible to ex vivo activation, considerable efforts were made to minimize this possibility; namely, blood withdrawal was performed using a 19-gauge needle to prevent perturbation of the granulocytes, acid-citrate dextrose was used as an anticoagulant as it results in less activation than heparin or EDTA,21 and hetastarch was used to remove red blood cells as sensitivity reactions have been reported after dextran use.17
Granulocyte blood kinetics and activation status of 111-Indium–labeled eosinophils. After reinjection of 111-Indium–labeled mixed leukocytes, 40-mL blood samples were taken. (A) Neutrophils (NEU) and (B) eosinophils (EO) were isolated from 40-mL blood samples using RoboSep before measurement of cell-associated radioactivity. (A-B) Data represent the mean ± SEM of 7 independent experiments. *P < .05, compared with 2-hour and 6-hour values (1-way ANOVA test). (C) Kinetics of eosinophils isolated after reinjection of purified 111-Indium–labeled eosinophils. Data are mean ± SEM of 6 independent experiments. Non-cell–associated radioactivity was measured by collecting plasma samples (○). Where error bars are not visible, the SEM lies within the symbols. (D) Representative flow cytometry and quantification of CD69, CD44, CD66b, and CD81 expression in freshly isolated whole blood eosinophils (WB), mixed leukocyte eosinophils (ML) ± 111-Indium tropolonate, and CD16 purified eosinophils (EO) ± 111-Indium tropolonate. Histograms represent WB (red line), ML (blue line), or EO (black line). Isotype-matched controls (gray fill) for WB, ML, or EO are shown from left to right. (E) Eosinophil shape change. Freshly isolated WB, and CD16 purified EO ± 111-Indium tropolonate were incubated with 10 ng/mL GM-CSF (GM) before assessment of shape change, as described in supplemental Methods. The means of cell size in forward scatter signal are shown. Data are the mean ± SEM of at least 3 independent experiments. *P < .05, compared with whole blood values (1-way ANOVA test). (F) Representative TEM images of eosinophils in WB, ML ± 111-Indium tropolonate, and EO ± 111-Indium tropolonate incubated with or without IL-5 (10 ng/mL). IL-5–treated eosinophils display signs of vacuolation (black arrows) and loss of granule integrity (white arrows). Sections were examined with a FEI, Tecnai G2 microscope operated at 120 kv using a 20 μm objective aperture. Digital images were captured with an AMT XR60B camera (Deben), saved in TIF format, and trimmed for publication using Microsoft Office Powerpoint. Original magnification ×3500.
Granulocyte blood kinetics and activation status of 111-Indium–labeled eosinophils. After reinjection of 111-Indium–labeled mixed leukocytes, 40-mL blood samples were taken. (A) Neutrophils (NEU) and (B) eosinophils (EO) were isolated from 40-mL blood samples using RoboSep before measurement of cell-associated radioactivity. (A-B) Data represent the mean ± SEM of 7 independent experiments. *P < .05, compared with 2-hour and 6-hour values (1-way ANOVA test). (C) Kinetics of eosinophils isolated after reinjection of purified 111-Indium–labeled eosinophils. Data are mean ± SEM of 6 independent experiments. Non-cell–associated radioactivity was measured by collecting plasma samples (○). Where error bars are not visible, the SEM lies within the symbols. (D) Representative flow cytometry and quantification of CD69, CD44, CD66b, and CD81 expression in freshly isolated whole blood eosinophils (WB), mixed leukocyte eosinophils (ML) ± 111-Indium tropolonate, and CD16 purified eosinophils (EO) ± 111-Indium tropolonate. Histograms represent WB (red line), ML (blue line), or EO (black line). Isotype-matched controls (gray fill) for WB, ML, or EO are shown from left to right. (E) Eosinophil shape change. Freshly isolated WB, and CD16 purified EO ± 111-Indium tropolonate were incubated with 10 ng/mL GM-CSF (GM) before assessment of shape change, as described in supplemental Methods. The means of cell size in forward scatter signal are shown. Data are the mean ± SEM of at least 3 independent experiments. *P < .05, compared with whole blood values (1-way ANOVA test). (F) Representative TEM images of eosinophils in WB, ML ± 111-Indium tropolonate, and EO ± 111-Indium tropolonate incubated with or without IL-5 (10 ng/mL). IL-5–treated eosinophils display signs of vacuolation (black arrows) and loss of granule integrity (white arrows). Sections were examined with a FEI, Tecnai G2 microscope operated at 120 kv using a 20 μm objective aperture. Digital images were captured with an AMT XR60B camera (Deben), saved in TIF format, and trimmed for publication using Microsoft Office Powerpoint. Original magnification ×3500.
Having established the kinetics of minimally manipulated eosinophils, we next imaged sites of eosinophil margination and uptake/disposal. Eosinophils were purified and labeled ex vivo before reinjection. Autoradiographs confirmed that the incorporated 111-Indium label was cell-associated (supplemental Figure 2). There were no significant differences in the expression of CD44, CD69, CD81, or shape change between the 2 cell populations, although CD66b was more highly expressed on the fully purified eosinophils (Figure 1D-E), as previously described.22 Furthermore, there was no alteration in the granule morphology of the 111-Indium–labeled purified eosinophils compared with whole blood eosinophils (Figure 1F; supplemental Figure 3). The purified eosinophils had a 45-minute recovery of 15% ± 2.6% and intravascular life span of 30 ± 2.7 hours (Figure 1C), which was highly comparable with the values obtained using labeled mixed leukocytes, again providing evidence that the purified eosinophils were nonactivated. A comparison of the peripheral eosinophil count and the labeled eosinophil recovery revealed a close correlation between the kinetics of labeled and nonlabeled cells, suggesting that the labeled eosinophils behave physiologically (supplemental Figure 4A). Correction of labeled eosinophil counts to the peripheral eosinophil numbers (supplemental Figure 4B) shows a near monoexponential decline in the number of circulating labeled eosinophils and suggests that the physiologic nocturnal eosinophilia reflects mobilization of eosinophils from the marginated blood pool.
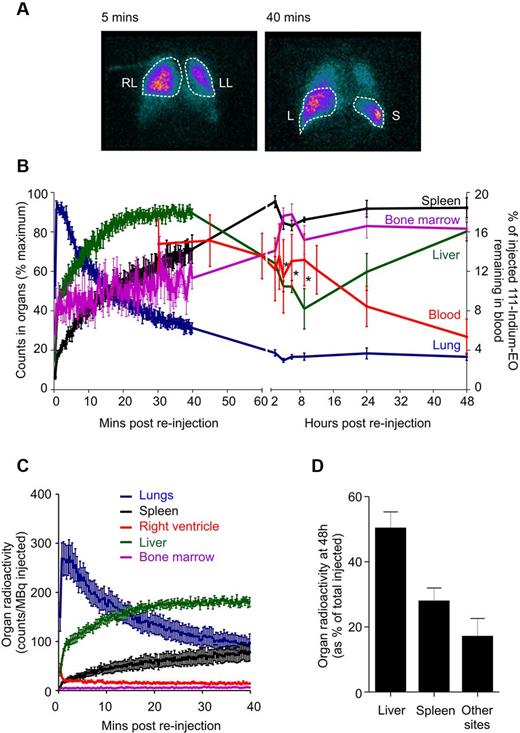
γ camera images of reinjected 111-Indium–labeled eosinophils demonstrated initial transit through the lungs, clearing to baseline by 40 minutes, with early accumulation in the liver and progressive accumulation in the spleen and bone marrow (Figure 2). Of note, the 9-hour blood recirculation peak coincided with a significant reduction of activity in the liver, and to a lesser extent, in the bone marrow signal. In contrast to neutrophils,23,24 very little signal was seen within the axial skeleton at any time point (Figure 2C). This implies that the bone marrow is not a major site for eosinophil disposal, with the principal sites being the liver and spleen (Figure 2D). Interestingly, after reinjection of eosinophils in a single splenectomized volunteer (supplemental Figure 5), there was increased eosinophil uptake in both the liver and also in the bone marrow; the distribution at 48 hours was 64% activity in the liver and 31% activity in other sites (principally composing the bone marrow).
γ camera quantification of 111-Indium–labeled eosinophils. (A) Anterior γ camera images at 5 minutes and 40 minutes after reinjection, showing accumulation in the right lung (RL), left lung (LL), liver (L), and spleen (S). (B) The distribution of radioactivity over 72 hours for the right lung (blue), liver (green), spleen (black), bone marrow (purple), and eosinophil recovery (red) after reinjection of 111-Indium–labeled eosinophils. (C) The distribution of radioactivity over 40 minutes for lungs, liver, spleen, bone marrow, and right ventricle after reinjection of 111-Indium–labeled eosinophils. (D) The final distribution of eosinophils within the liver and spleen at 48 hours. Radioactivity in other sites was calculated by subtracting the liver, spleen, lung, and blood activity values from the total radioactivity injected. The value obtained may reflect regions, such as the bone marrow, which are difficult to fully quantify on images of the upper body alone. Data represent the mean ± SEM of 6 independent experiments. *P < .05, compared with 40-minute liver values (1-way ANOVA test).
γ camera quantification of 111-Indium–labeled eosinophils. (A) Anterior γ camera images at 5 minutes and 40 minutes after reinjection, showing accumulation in the right lung (RL), left lung (LL), liver (L), and spleen (S). (B) The distribution of radioactivity over 72 hours for the right lung (blue), liver (green), spleen (black), bone marrow (purple), and eosinophil recovery (red) after reinjection of 111-Indium–labeled eosinophils. (C) The distribution of radioactivity over 40 minutes for lungs, liver, spleen, bone marrow, and right ventricle after reinjection of 111-Indium–labeled eosinophils. (D) The final distribution of eosinophils within the liver and spleen at 48 hours. Radioactivity in other sites was calculated by subtracting the liver, spleen, lung, and blood activity values from the total radioactivity injected. The value obtained may reflect regions, such as the bone marrow, which are difficult to fully quantify on images of the upper body alone. Data represent the mean ± SEM of 6 independent experiments. *P < .05, compared with 40-minute liver values (1-way ANOVA test).
These studies reveal, for the first time, the kinetics of eosinophil circulation in healthy human subjects and demonstrate their physiologic uptake in, and release from, the reticuloendothelial system. These studies also provide the basis for future noninvasive quantification of eosinophil accumulation in tissues.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rosalind Simmonds for recruiting study participants; Katrin Eitel for assistance with sample collection; Dr Jeremy Skepper (Multi Imaging Centre, Department of Physiology, Development and Neuroscience, University of Cambridge) for TEM assistance; the staff at the Nuclear Medicine Department at Addenbrooke's Hospital and the Wellcome Trust Clinical Research Facility, Cambridge; Cambridge Biomedical Research Center Core Biochemistry Assay Laboratory; and the National Institute for Health Research, through the Comprehensive Clinical Research Network.
This work was supported by Asthma United Kingdom (08/11), the Medical Research Council (grant MR/J00345X/1), and Cambridge National Institute for Health Research Biomedical Research Center.
Authorship
Contribution: N.F. performed experiments, analyzed the data, and wrote the manuscript, with contributions from the other authors as appropriate; N.R.S., C.L., and P.R. performed experiments; S.H. and C.S. analyzed data; C.K.S., K.S., and K.K.B. coordinated the labeling and imaging study; A.M.C. analyzed data and wrote the manuscript; E.R.C. and A.M.P. conceived and designed the study and wrote the manuscript; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edwin R. Chilvers, Department of Medicine, University of Cambridge School of Clinical Medicine, Addenbrooke's and Papworth Hospitals, Cambridge, CB2 0QQ, United Kingdom; e-mail: erc24@cam.ac.uk.
References
Author notes
A.M.P., A.M.C. and E.R.C. are joint senior authors.