Abstract
TGF-β–activated kinase 1 (TAK1), a member of the MAPK kinase family, plays a key role in B-cell growth and development. In the present study, we examined the potential role of TAK1 as a therapeutic target for lymphoma. Here, we show that the active phosphorylated form of TAK1 is abundantly expressed in a panel of lymphoma cell lines, including mantle cell, anaplastic large cell, and Hodgkin lymphoma cell lines. Silencing TAK1 expression via the use of siRNA inhibited the activation of NF-κB and p38 and induced apoptosis in lymphoma cell lines. Moreover, submicromolar concentrations of AZ-TAK1, a novel ATP-competitive small molecule inhibitor of TAK1, dephosphorylated TAK1, p38, and IκB-α in lymphoma cell lines. These molecular events were associated with the release of cytochrome c into the cytosol, down-regulation of X-linked inhibitor of apoptosis, activation of caspase 9, and induction of apoptosis. We also demonstrate that primary lymphoma cells express TAK1 and pTAK1 and were sensitive to AZ-TAK1–mediated cell death. Collectively, our data demonstrate an essential role for TAK1 in regulating critical survival mechanisms in lymphoma and suggest that it may serve as a therapeutic target.
Introduction
TGF-β–activated kinase 1 (TAK1) is a serine/threonine kinase that was identified in 1995 as a member of the MAPK kinase family (MAP3K7).1 TAK1 is activated by TGF-β and by a variety of cytokines, including TNF, IL-1, CD40 ligand, toll-like receptors, and T- and B-cell receptors.2-4 On receptor activation, TAK1 binds to adaptor proteins and subsequently activates key downstream kinases such as IκK, p38 MAPK, and c-jun N-terminal kinase. In turn, this step leads to activation of NF-κB and activator protein–1 transcription factors that modulate the expression of a variety of inflammatory cytokines.5 Collectively, these cellular events enable TAK1 to play a key role in regulating inflammation, immunity, and cell death in a variety of cell types.6,7
Within the hematopoietic system, TAK1 plays an important role in promoting T-cell development and B-cell maturation, function, and survival.4,6,8,9 B cell–specific deletion of TAK1 has been shown to markedly decrease marginal zone B cells in mice and to be associated with impaired B-cell proliferation and survival.4 TAK1-deficient B cells also failed to activate NF-κB and c-jun N-terminal kinase in response to B-cell receptor stimulation.4 However, the expression and function of TAK1 in lymphoid malignancies, especially those known to aberrantly express activated NF-κB, remain unclear.10 Here, we show that TAK1 and its active phosphorylated form (pTAK1) are abundantly expressed in a variety of primary and cultured lymphoma cells. Furthermore, inhibiting TAK1 via the use of siRNA or the small-molecule inhibitor AZ-TAK1 inactivated NF-κB, down-regulated p38, and activated the intrinsic caspase pathway, resulting in profound induction of apoptosis. Our data demonstrate a pivotal role for TAK1 in regulating lymphoma cell survival and suggest that it may be a therapeutic target for lymphoma.
Methods
Cell lines, primary lymphoma samples, and cell culture
The human Hodgkin and Reed-Sternberg–derived cell lines HD-LM2, L-428, and KM-H2 were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures. All cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL), 1% l-glutamine, and penicillin/streptomycin in a humid environment of 5% CO2 at 37°C. MCL lines (Jeko-1, Mino, and SP53) and anaplastic large-cell lymphoma cell lines (Karpas-299, SUP-M2, and SU-DHL-1) were cultured in a similar way. The phenotypes and genotypes of these cell lines have been previously published.11-16 Peripheral blood samples were obtained from 5 patients with MCL. Patient samples had been deposited in The University of Texas MD Anderson Cancer Center Lymphoma Blood Bank at the time of diagnosis or relapse. All patients and healthy volunteers had previously provided consent for donation of peripheral blood samples in accordance with The University of Texas MD Anderson Cancer Center Institutional Review Board guidelines.
Reagents, antibodies, and recombinant proteins
The TAK-1 inhibitor (AZ-TAK1) was obtained from AstraZeneca. For Western blot experiments, antibodies to pTAK1 (Thr187), p38, phosphorylated p38, caspase 8, caspase 9 and cleaved caspase 3, AKT, pAKT (Ser473), pAKT (Thr308), ERK, pERK, p65 NF-κB, IκB, pIκB, SMAC/Diablo, cytochrome c, TRAF, X-linked inhibitor of apoptosis (XIAP), Syk, pSyk, Btk, and pBtk were purchased from Cell Signaling Technology. Antibody to XIAP was purchased from Santa Cruz Biotechnology. Antibody to β-actin was from Sigma-Aldrich.
In vitro proliferation assay
Cells were cultured in 12-well plates at a concentration of 0.5 × 106 cells/mL. Cell viability was assessed by use of the nonradioactive cell proliferation MTS [3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium] assay with CellTiter96AQueous One Solution Reagent (Promega), as previously published.17 To summarize, 80 μL of cell suspension with 20 μL of CellTiter96AQueous One Solution Reagent was incubated in 96-well plates for 1 hour at 37°C and 5% CO2, and formazan absorbance was measured at 490 nm on a μQuant plate reader equipped with Gen5 software (Biotek Instruments). Each measurement was made in triplicate, and the mean value was determined. Results represent the mean value of 3 independent experiments.
Western blot analysis
Total cellular proteins were extracted by incubation in lysis buffer (Cell Signaling Technology) for 40 minutes on ice and then centrifuged to remove cellular debris. The protein in the resulting supernatant was quantified. Then the protein was diluted 1:2 in protein sodium dodecyl sulfate loading buffer (Cell Signaling Technology) and heated to 95°C for 5 minutes. A total of 30 μg of protein was loaded onto 12% tris-HCl sodium dodecyl sulfate polyacrylamide electrophoresis Ready Gels (Bio-Rad), transferred to a nitrocellulose transfer membrane (Bio-Rad), and detected by the use of SuperSignalWest Dura Extended Duration Substrate (Pierce Chemical), as previously described.18,19
Cytoplasmic and nuclear protein extraction
Proteins from cytoplasmic and nuclear cell fractions were extracted with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific). To summarize, cells were incubated for 10 minutes on ice with the cytoplasmic extraction reagent I-II and then centrifuged. The proteins in the resulting supernatant represent the cytoplasmic extract. Nuclear extract was obtained by incubating cell pellets with Nuclear Extraction Reagent for 40 minutes. Cell suspensions were placed on ice and vortexed every 10 minutes for a total of 40 minutes. After centrifugation, the supernatants represent the nuclear extract. The proteins in the resulting cytoplasmic/nuclear extracts were quantified by with the bicinchoninic acid method (Pierce Chemical) according to the manufacturer's instructions.
Preparation of cytosolic and membrane fractions
Mitochondrial and cytosol fractions were isolated with the Mitochondria/Cytosol Fractioning Kit (BioVision). Cells were washed with ice-cold phosphate-buffered saline, resuspended in 1 mL of Cytosol Extraction Buffer Mix containing dithiothreitol and protease inhibitors, and incubated on ice for 10 minutes. Cells were homogenized by 30 passes with a Dounce tissue grinder. Cell homogenates were incubated on ice for 10 minutes and then centrifuged at 700g for 10 minutes at 4°C. Protein concentration of the resulting postnuclear fraction (supernatant) was collected, and cells were further centrifuged at 10 000g for 40 minutes at 4°C. The resulting supernatant was used as the cytosolic fraction and the pellet as the membrane fraction. The membrane fraction was resuspended and homogenized in 100 μL of Mitochondrial Extraction Buffer Mix to obtain the mitochondrial protein lysates.
Flow cytometry
Apoptosis was determined by Annexin V–FITC Apoptosis Detection Kit, performed according to the manufacturer's instructions (BD Pharmingen). Data were acquired a FACSCalibur flow cytometer (BD Biosciences). Results were obtained by analyzing data with FlowJo Version 7.6.1 software (TreeStar) Results represent the mean value of 3 independent experiments.
Evaluation of mitochondrial membrane potential
Mitochondrial membrane potential (ΔΨ) was evaluated by using the mitochondrial specific fluorescent probe 5,5′, 6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolycarbocyanine iodide (JC-1), performed according to the manufacturer's instructions. (Trevigen). Data were acquired by FACSCalibur flow cytometer (BD Biosciences). Results were obtained by analyzing data with FlowJo Version 7.6.1 software (TreeStar). Results represent the mean value of 3 independent experiments.
Silencing TAK-1 gene expression by siRNA
Three sets of double-stranded siRNA for TAK-1 and of silencer-negative siRNA (as a control) were purchased from Invitrogen. The sequences of the siRNAs are available on request. The MCL cell lines (Jeko-1, Mino, and SP53) were plated at 1 × 106 cells/mL in 12-well plates. Double-stranded siRNAs (2μM) were transfected with the Nucleofector Kit (Lonza). For MCL cell line Jeko-1, we used Nucleofector Solution V and the transfection program X-001, for Mino and SP-53 we used Nucleofector Solution T and the transfection program T-001. Cells were harvested after 48 hours and subjected to Western blot analysis. This protocol gave a transfection efficiency of 70%-80%.
Immunohistochemistry and immunofluorescence
Immunohistochemical methods were performed by the use of routinely processed paraffin-embedded tissue specimens from tumors and cytospin smears from mantle cell lines (Mino, P53, and Jeko) as previously described.20 The slides were incubated for 1.5 hours at room temperature with antitotal-TAK1 antibody (dilution 1:150; Abcam). All tissue sections underwent heat-induced epitope retrieval in pH 6.0 citrate buffer (Dako) followed by cooling-down period of 20 minutes. Endogenous peroxidase reaction was blocked by 3% H2O2 solution for 10 minutes. To avoid nonspecific binding of primary antibodies, serum-free blocking solution (Dako) was applied for 40 minutes at room temperature.
Detection was performed with the LSAB plus streptavidin HRP system (Dako). Immunofluorescence labeling was also performed with cytospin smears from mantle cell lymphoma (MCL) cell lines as well as paraffin-embedded tissue section specimens of malignant lymphomas as previously described.21 For the cytospin specimens, the slides were incubated for 1 hour at room temperature with an antiphospho (p)TAK1 antibody (dilution 1:200; Abcam). For the paraffin-embedded tissue sections, the slides were incubated overnight at 4°C using the same antibody (dilution 1:50). The tissue sections underwent heat-induced epitope retrieval in pH 6.0 citrate buffer (Dako) followed by a cooling-down period of 20 minutes. Alexa Fluor 594 (red) goat antibody IgG conjugate (Molecular Probes) was used as secondary antibody. The nuclei were counterstained with 4′-6′ diamidino-2-phenylindole.
Sections from an infiltrating ductal breast carcinoma and from a diffuse large B-cell lymphoma cell line, SUDHL2, were used as positive and negative controls, respectively. The Jurkatt cell line was also used as a positive control for pTAK. In addition, the baseline levels of expression of total and pTAK1 were assessed in a reactive lymph node. Protein expression levels for both, total-TAK1, and pTAK1 were scored as negative, weakly positive (+), and strongly positive (++), depending on the staining signal intensity in comparison with the positive control; if greater or equal, then expression was considered high and if lower, then expression was considered low.
TAK1 kinase assay
An in vitro kinase assay was used to measure the ability of AZ-TAK1 to inhibit the kinase activity of TAK1 toward a recombinant protein substrate. The TAK1 kinase domain and TAB protein were coexpressed in baculovirus and purified by FLAG tag. The purified protein complex and biotinylated full-length kinase dead substrate (BT-MKK6kd) were then used in a 384-well automated ALPHA (Amplified Luminescent Proximity Homogeneous Assay) screen assay. Specifically, BT-MKK6kd substrate, TAKTAB enzyme complex, and reaction buffer were mixed and aliquoted into a 384-well plate containing a dilution of AZ-TAK1 and appropriate controls. ATP buffer was then added to initiate the reaction. After 1 hour, an alphascreen detection mixture, containing alphascreen acceptor and donor beads, antiphospho-MKK6 antibody, and EDTA, was added to stop the reaction. This allowed the streptavidin-coated donor beads to bind to the biotin-labeled MKK6kd substrate and the protein A coated acceptor beads to bind to the antiphospho-MKK6 antibody. When the antiphospho-MKK6 antibody bound to the phosphorylated MKK6kd substrate, the 2 beads were brought in close proximity of each other, resulting in the generation of a luminescent signal that was quantified using a Perkin-Elmer Envision plate reader. Data were analyzed to determine the mean IC5s for AZ-TAK1.
Modeling studies of AZ-TAK1
Tak1 crystal structure was solved in house (Proteros Biostructures GmbH, unpublished work, August 10, 2008), and the structure was prepared by use of the Maestro 8.5 protein preparation wizard (Schrödinger); waters were deleted, bond orders assigned, and hydrogens added. The ligand was prepared with the use of default settings in LigPrep Version 2.5 (Schrödinger), and docking studies were performed with the program Glide.22
p65 translocation assay
In summary, HeLa cells were plated in 96-well plates and after pretreatment for 1 hour with AZ-TAK1, cells were stimulated for 20 minutes with 2 ng/mL IL-1. The cells were then fixed with paraformaldehyde, thereafter permeabilized, and stained with the antibodies. Fluorescent immunolabeling was used to monitor changes in the nuclear translocation of NF-κB p65, phospho-cJun, and phospho-p38. NF-κB p65 was obtained from Santa Cruz Biotechnology, and phospho-p38 Ab, and phospho-cJun were obtained from Cell Signaling technologies. Alexa Fluor dye conjugated secondary Abs were obtained from Molecular Probes. Cellular screening, data acquisition, and data interpretation were conducted on the Array Scan HCS Reader (Cellomics).
ELISA
MCL cell lines were incubated in RMPI with or without FBS for 6-24-48 hours, before supernatants were collected and examined for TNF-α production by ELISA (R&D Systems), according to the manufacturer's instructions and as previously published.17 Each experiment was performed in triplicate, and results represent the mean value from 3 different experiments.
Mass spectroscopy–based approach evaluating single-nucleotide polymorphisms
A mass spectroscopy-based approach evaluating single nucleotide polymorphisms was used to detect the KRAS mutation. PCR and extension primers for KRAS were designed with Sequenom. Assay design. Chips were run in duplicate on a Sequenom Mass-Array MALDI-TOF MassArray system. Sequenom Typer Software and visual inspection were used to inspect mass spectra.23
Statistical analyses
The 2-tailed Student t test was used to estimate the statistical significance of differences in results among the 3 experiments. Significance was set at P < .05.
Results
Expression of TAK1 in lymphoma
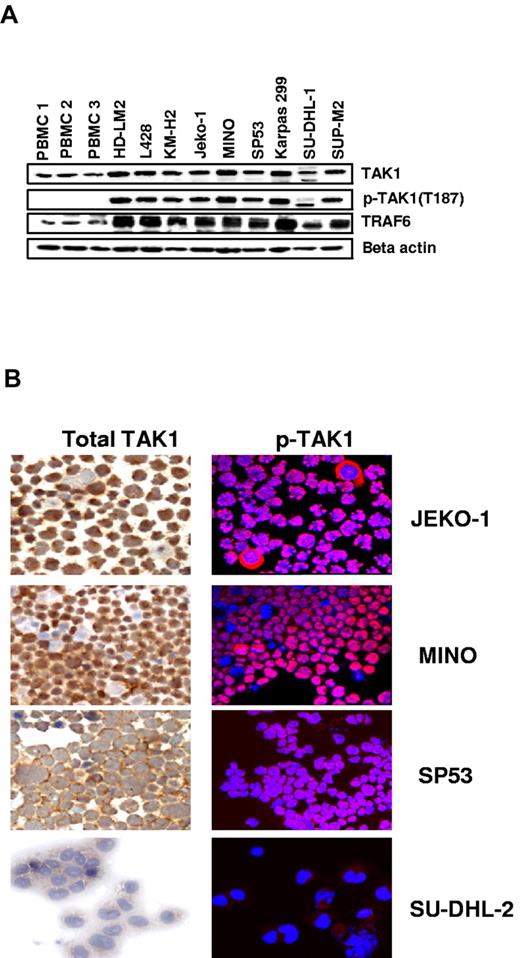
First, we examined the pattern of TAK1 expression in a panel of lymphoid cell lines derived from Hodgkin lymphoma (HD-LM2, L428, and KM-H2), MCL (Jeko-1, Mino, and SP53), and anaplastic large-cell lymphoma (Karpas-299, SU-DHL1, and SUP-M2) and compared the results with those of PBMCs from healthy persons (Figure 1A). Cellular stress-induced activation of TAK1 involves phosphorylation of the activation loop when TAK1 is in a signaling complex with TAB1 and TAB2.24 Notably, transautophosphorylation of Thr187 appears to be correlated with endogenous TAK1 kinase activity.25
Expression of TAK1 in lymphoma cell lines. (A) Lymphoma cell lines have greater expression of TAK1 and pTAK1 compared with PBMCs from healthy donors. TRAF6, which is frequently required for TAK1 activation, and β-actin were used as controls. (B) Immunohistochemical analysis of TAK1 and pTAK1 expression in the cytospins of MCL lines demonstrating a cytoplasmic distribution pattern for pTAK1.
Expression of TAK1 in lymphoma cell lines. (A) Lymphoma cell lines have greater expression of TAK1 and pTAK1 compared with PBMCs from healthy donors. TRAF6, which is frequently required for TAK1 activation, and β-actin were used as controls. (B) Immunohistochemical analysis of TAK1 and pTAK1 expression in the cytospins of MCL lines demonstrating a cytoplasmic distribution pattern for pTAK1.
We therefore sought to examine the level of TAK1 phosphorylation by using an antibody directed against pThr187 (hereafter referred to as pTAK1). PBMCs from 3 healthy donors weakly expressed TAK1 but did not express the active phosphorylated form of pTAK1 (Thr187). In contrast, TAK1, pTAK1, and its binding partner (TRAF6) were expressed in all MCL and Hodgkin lymphoma cell lines but expressed to a lesser extent in the anaplastic large-cell lymphoma cells. The expression pattern and cellular localization of TAK1 and pTAK1 in the MCL cells were then examined by the use of fluorescence microscopy. Both TAK1 and pTAK1 were predominantly expressed in the cytoplasm and were observed in ∼ 90% of cells, but was also weakly expressed in some of the nuclei (Figure 1B).
To investigate the mechanism of TAK1 activation in MCL cell lines, we examined the levels of TAK1 and pTAK1 in normal culture system and under starved culture conditions. For these experiments, we incubated Jeko-1, Mino, and SP53 for 3-6-24 hours in RPMI with and without FBS 20%. Western blot results showed no difference in TAK1 and pTAK1 expression between starved and not starved cells. Both Jeko-1 and Mino cells secreted TNF-α in the supernatants, which may have contributed to the aberrant activation of TAK1 (supplemental Figure 3, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Silencing TAK1 gene expression inhibits NF-κB and p38 activation and induces apoptosis in MCL
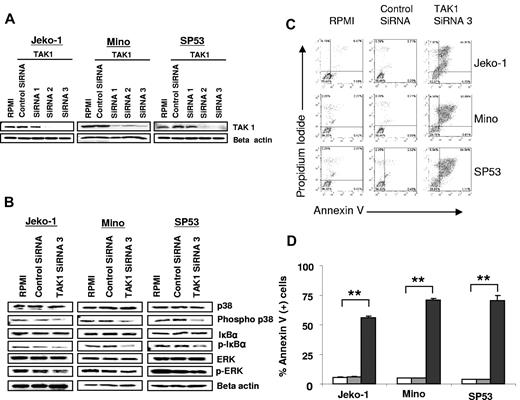
TAK1 is essential for p38 and NF-κB activation and therefore plays a key role in regulating inflammation, immunity, and survival, but its function in lymphoid malignancies remains unknown.3-7 We examined the molecular and functional consequences of TAK1 gene silencing in MCL cells, which are known to rely on activated NF-κB pathway to promote their survival. Using 3 different sets of double-stranded siRNAs,1-3 we found that the use of siRNAs 2 and 3 effectively silenced the expression of TAK1 in all cell lines, and therefore we used siRNA3 in subsequent experiments (Figure 2A). TAK1 gene silencing resulted in inactivation of p38 and NF-κB, as indicated by the dephosphorylation of p38 and IκB. In addition, TAK1 silencing slightly reduced ERK phosphorylation, especially in the Jeko-1 cells (Figure 2B). These molecular changes were associated with induction of apoptosis in up to 71% of the lymphoma cells within 48 hours (Figure 2C-D). Collectively, these data demonstrate that TAK1 plays a critical role in regulating lymphoma cell survival and suggest that TAK1 may serve as a therapeutic target in lymphoma.
Biologic and molecular consequences of silencing TAK1 expression in lymphoma. (A) A representative experiment of TAK1 gene silencing with the use of 3 different siRNAs in lymphoma cell lines. TAK1 protein levels were determined by Western blot after 48 hours of transfection. Similar results were obtained in 2 additional independent experiments. (B) TAK-1 gene silencing decreased the phosphorylation of p38 and IκBα without affecting ERK phosphorylation. Results are shown after 48 hours of transfection. (C) TAK1 gene silencing resulted in induction of apoptosis in lymphoma cell lines, as determined by the annexin V/propidium iodide staining method and FACS analysis. This is a representative experiment performed after 48 hours of transfection with TAK1 siRNA or control siRNA. (D) Summary results of TAK1 siRNA–induced cell death. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). **P < .005. Open bars indicate RPMI; gray bars, control siRNA; and black bars, TAK1 siRNA3.
Biologic and molecular consequences of silencing TAK1 expression in lymphoma. (A) A representative experiment of TAK1 gene silencing with the use of 3 different siRNAs in lymphoma cell lines. TAK1 protein levels were determined by Western blot after 48 hours of transfection. Similar results were obtained in 2 additional independent experiments. (B) TAK-1 gene silencing decreased the phosphorylation of p38 and IκBα without affecting ERK phosphorylation. Results are shown after 48 hours of transfection. (C) TAK1 gene silencing resulted in induction of apoptosis in lymphoma cell lines, as determined by the annexin V/propidium iodide staining method and FACS analysis. This is a representative experiment performed after 48 hours of transfection with TAK1 siRNA or control siRNA. (D) Summary results of TAK1 siRNA–induced cell death. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). **P < .005. Open bars indicate RPMI; gray bars, control siRNA; and black bars, TAK1 siRNA3.
Identification of AZ-TAK1, a novel ATP-competitive inhibitor of TAK1 in vitro
AZ-TAK1 was discovered after a small molecule-based lead identification program at AstraZeneca. Modeling studies of AZ-TAK1 suggest that the compound binds to the hinge region of the ATP binding site of Tak1 via correlated hydrogen bonds with the hinge Ala81. A crystal structure of similar compounds in Tak1 (Proteros Biostructures GmbH, unpublished work, August 10, 2008) confirm the predicted binding mode of AZ-TAK1 (Figure 3A). The inhibitory activity of AZ-TAK1 against TAK1 (MAP3K7) was evaluated by the use of a complex of full-length recombinant human TAK1 and its activating partner TAB1 and full-length kinase dead MKK6 as a substrate. Using this assay at Km ATP (10μM), we found that the IC50 for AZ-TAK1 was 8.0 ± 0.05 nmol/L (Figure 3B). Enzymatic reactions performed with 2000μM of ATP resulted in an IC50 increase to 177 ± 0.05 nmol/L, indicating that AZ-TAK1 binds competitively with ATP (supplemental Figure 1). The selectivity of AZ-TAK1 was evaluated in a panel of 30 kinases representing the major kinase families (Figure 3C). The most sensitive kinases in addition to TAK1 were CMGC-family kinases HIPK2, CDK9, and GSK3β, with IC50 values estimated as 3 nmol/L, 9 nmol/L, and 19 nmol/L, respectively (Figure 3C).
Identification of the small molecule inhibitor AZ-TAK1. (A) Docked pose of AZ-TAK1 in TAK1 crystal structure. Binding mode of AZ-TAK1 (1-(2-(3-ethyl-5-(5-fluoro-4-(imidazo[1,2-b]pyridazin-3-yl)pyrimidin-2-ylamino)phenoxy)ethyl)piperidin-4-ol) is consistent with in house cocrystal structures of close analogs of AZ-TAK1 (Proteros Biostructures GmbH, unpublished work, August 10, 2008). AZ-TAK1 adopts a U-shaped conformation in TAK1, with the imidazopyridazine heterocycle packing atop C148 and aminopyrimidine making 2 hydrogen bond interactions with the hinge (A81). The 3-ethyl moiety points toward the sugar pocket and the 4-hydroxypiperidine is out in the solvent channel. (B) The ability of AZ-TAK1 to inhibit TAK1 kinase activity was measured in vitro with the use of a complex of recombinant TAK1 and TAB1 and the substrate MKK6kd. Data shown are representative of 3 independent assays. (C) Selectivity of AZ-TAK1 against a panel of 30 kinases.
Identification of the small molecule inhibitor AZ-TAK1. (A) Docked pose of AZ-TAK1 in TAK1 crystal structure. Binding mode of AZ-TAK1 (1-(2-(3-ethyl-5-(5-fluoro-4-(imidazo[1,2-b]pyridazin-3-yl)pyrimidin-2-ylamino)phenoxy)ethyl)piperidin-4-ol) is consistent with in house cocrystal structures of close analogs of AZ-TAK1 (Proteros Biostructures GmbH, unpublished work, August 10, 2008). AZ-TAK1 adopts a U-shaped conformation in TAK1, with the imidazopyridazine heterocycle packing atop C148 and aminopyrimidine making 2 hydrogen bond interactions with the hinge (A81). The 3-ethyl moiety points toward the sugar pocket and the 4-hydroxypiperidine is out in the solvent channel. (B) The ability of AZ-TAK1 to inhibit TAK1 kinase activity was measured in vitro with the use of a complex of recombinant TAK1 and TAB1 and the substrate MKK6kd. Data shown are representative of 3 independent assays. (C) Selectivity of AZ-TAK1 against a panel of 30 kinases.
Using an IL-1–induced HeLa cell line model, we found that AZ-TAK1 inhibited the phosphorylation of p38 and c-Jun and prevented the nuclear translocation of p65, a hallmark of NF-κB activation, within 1 hour of incubation (supplemental Figure 1A-B). Moreover, we performed additional experiments to evaluate the effect of AZ-TAK1 and TAK-1 silencing SiRNA on SYK and BTK (supplemental Figure 2A-B). AZ-TAK1 decreased the phosphorylation of Syk and BTK in the 3 MCL cell lines (supplemental Figure 2A). Furthermore, TAK1 silencing by siRNA also was associated with a decrease in Syk phosphorylation and BTK phosphorylation, which were more prominent in the SP53 cells (supplemental Figure 2B). These data may indeed suggest that greater concentrations of AZ-TAK1 may also inhibit Syk, which may have contributed to the efficacy of the small molecule. However, siRNA silencing of TAK1 also was associated with a decrease in pSyk and pBTK, which may suggest that TAK1 inhibition may lead to inhibition of BCR signaling by modulating the expression of cytokines and growth factors that can induce Syk and BTK phosphorylation.
Pharmacologic inhibition of TAK1 by the small-molecule inhibitor AZ-TAK1 induces apoptosis in lymphoma cell lines
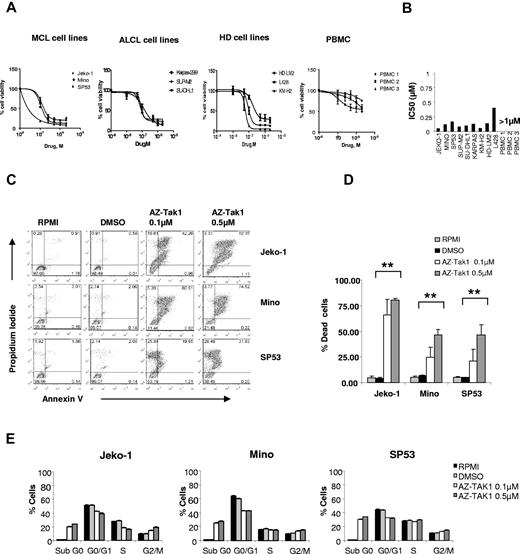
We examined the biologic activity of AZ-TAK1 in a panel of 9 lymphoma cell lines and PBMCs isolated from 3 healthy donors. AZ-TAK1 potently inhibited lymphoma cell line proliferation and induced apoptosis in a dose- and time-dependent manner. In 8 of the 9 cell lines, the IC50 of AZ-TAK1 was < 0.25μM. In contrast, AZ-TAK1 had no significant effect on PBMC survival (Figure 4A-B). The antiproliferative effect of AZ-TAK1 was mainly a result of the induction of apoptosis, as determined by dual staining with Annexin V and propidium iodide and FACS analysis (Figure 4C-D). After 48 hours of incubation, 0.1μM AZ-TAK1 induced apoptosis in 28%, 24%, and 74% of Mino, SP53, and Jeko cells, respectively. The induction of apoptosis increased to 42%, 46%, and 86% when cells were incubated with 0.5μM concentration. Moreover, the antiproliferative activity of AZ-TAK1 was not associated with a significant effect on cell-cycle fractions (Figure 4E).
Molecular and biologic consequences of pharmacologic inhibition of TAK1 in lymphoma cell lines using AZ-TAK1. (A) Cell lines of Hodgkin lymphoma origin (HD), MCL, or anaplastic large cell lymphoma (ALCL) were incubated with increasing concentrations (0.1, 0.5, 1, and 2μM) of the AZ-TAK1 for 72 hours before cell viability was determined with the MTS assay. PBMCs from 3 healthy donors were included for comparison. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). (B) IC50 values for AZ-TAK1 in lymphoma cell lines and PBMCs at 72 hours (C) Effect of AZ-TAK1 on induction of apoptosis. The MCL cell lines Jeko-1, Mino, and SP53 were incubated with dimethyl sulfoxide (DMSO; 0.1%) or AZ-TAK1 (0.1 or 0.5μM) for 48 hours before apoptosis was measured using the annexin V/PI dual staining method and FACS analysis. (D) Summary results of AZ-TAK1–induced cell death (PI and annexin V–positive cells) from 3 independent experiments. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). **P < .005. (E) Effect of AZ-TAK1 on cell-cycle analysis. The MCL cell lines Jeko-1, Mino and SP53 were incubated with DMSO (0.1%) or AZ-TAK1 (0.1 or 0.5μM) for 24 hours before cell-cycle analysis was performed using the PI staining method by FACS analysis.
Molecular and biologic consequences of pharmacologic inhibition of TAK1 in lymphoma cell lines using AZ-TAK1. (A) Cell lines of Hodgkin lymphoma origin (HD), MCL, or anaplastic large cell lymphoma (ALCL) were incubated with increasing concentrations (0.1, 0.5, 1, and 2μM) of the AZ-TAK1 for 72 hours before cell viability was determined with the MTS assay. PBMCs from 3 healthy donors were included for comparison. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). (B) IC50 values for AZ-TAK1 in lymphoma cell lines and PBMCs at 72 hours (C) Effect of AZ-TAK1 on induction of apoptosis. The MCL cell lines Jeko-1, Mino, and SP53 were incubated with dimethyl sulfoxide (DMSO; 0.1%) or AZ-TAK1 (0.1 or 0.5μM) for 48 hours before apoptosis was measured using the annexin V/PI dual staining method and FACS analysis. (D) Summary results of AZ-TAK1–induced cell death (PI and annexin V–positive cells) from 3 independent experiments. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). **P < .005. (E) Effect of AZ-TAK1 on cell-cycle analysis. The MCL cell lines Jeko-1, Mino and SP53 were incubated with DMSO (0.1%) or AZ-TAK1 (0.1 or 0.5μM) for 24 hours before cell-cycle analysis was performed using the PI staining method by FACS analysis.
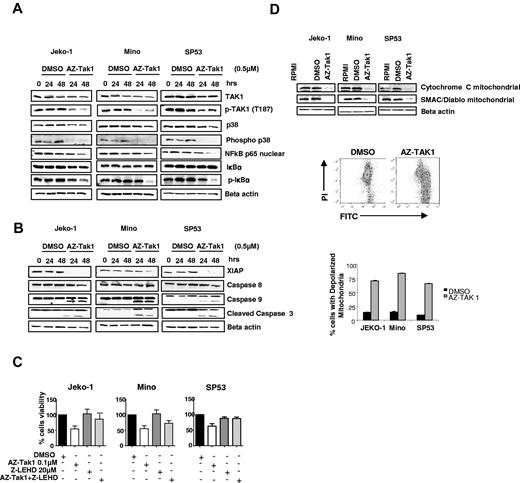
At the molecular level, AZ-TAK1 induced similar changes to those observed with TAK1 silencing experiments. As shown in Figure 5A, AZ-TAK1 inhibited the phosphorylation of TAK1, p38, and IκBα (also shown in supplemental Figure 4) in addition to decreasing the nuclear content of p65, indicative of NF-κB inactivation. AZ-TAK1 activated the intrinsic caspase pathway by down-regulating XIAP protein expression and cleavage of caspases 9 and 3 (Figure 5B). Furthermore, the caspase 9 inhibitor Z-LEHD-FMK either partially (Jeko-1, Mino) or completely (SP53) inhibited AZ-TAK1–induced cell death (Figure 5C). Moreover, AZ-TAK1–mediated activation of caspase 9 was associated with the release of cytochrome c from the mitochondria into the cytosol, which was augmented by the release of SMAC/Diablo from the mitochondria (Figure 5D). Finally, we examined the effect of AZ-TAK1 on mitochondria membrane potential by using the fluorescent membrane-permeant JC-1 dye staining method and flow cytometric analysis (Figure 5D). AZ-TAK1 increased mitochondrial depolarization, indicated by a decrease in the red/green fluorescence intensity ratio. Cells with depolarized mitochondria accounted for 75% of total cell number when treated with AZ-TAK1 compared with control (8%; Figure 5D). Collectively, these data demonstrate that AZ-TAK1 induces cell death in lymphoma cells by releasing cytochrome c and SMAC/Diablo from the mitochondria, down-regulating XIAP, and activation of caspase 9 and 3.
Molecular mechanisms of AZ-TAK1 antiproliferative activity lymphoma. (A) MCL cell lines were incubated with medium or 0.5μM of AZ-TAK1 for 24-48 hours. Whole-cell lysates were examined by Western blot for changes in intracellular proteins. AZ-TAK1 decreased TAK1 and p38 phosphorylation, indicative of their inactivation. AZ-TAK1 inactivated NF-κB, as indicated by the decrease in nuclear p65 and inhibition of IκB phosphorylation. (B) AZ-TAK1 down-regulated XIAP and activated caspase 9 and caspase 3. (C) AZ-TAK1–induced cell death was either partially or completely blocked by the caspase 9 inhibitor Z-LEHD-FMK. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). (D) AZ-TAK1 released cytochrome c and SMAC/Diablo from the mitochondria in MCL cell lines (top). Western blot analysis was performed on subcellular mitochondrial fractions after incubation with AZ-TAK1 (0.5μM) for 48 hours. Cytofluorimetric analysis of MCL cell lines mitochondrial potential, as assessed with the mitochondrial specific cationic dye (middle), as described in “Evaluation of mitochondrial membrane potential.” In this representative experiment, treatment with AZ-TAK resulted in mitochondria depolarization in 75% of the cells, compared with control 8% in DMSO-treated cells. Summary of 3 independent experiments demonstrating the effect of AZ-TAK1 on mitochondria membrane polarization (bottom). Each value represents the percentage of cells with depolarized mitochondria. Cells were incubated for 12 hours in the absence (DMSO) or presence of AZ-TAK1 0.5μM.
Molecular mechanisms of AZ-TAK1 antiproliferative activity lymphoma. (A) MCL cell lines were incubated with medium or 0.5μM of AZ-TAK1 for 24-48 hours. Whole-cell lysates were examined by Western blot for changes in intracellular proteins. AZ-TAK1 decreased TAK1 and p38 phosphorylation, indicative of their inactivation. AZ-TAK1 inactivated NF-κB, as indicated by the decrease in nuclear p65 and inhibition of IκB phosphorylation. (B) AZ-TAK1 down-regulated XIAP and activated caspase 9 and caspase 3. (C) AZ-TAK1–induced cell death was either partially or completely blocked by the caspase 9 inhibitor Z-LEHD-FMK. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). (D) AZ-TAK1 released cytochrome c and SMAC/Diablo from the mitochondria in MCL cell lines (top). Western blot analysis was performed on subcellular mitochondrial fractions after incubation with AZ-TAK1 (0.5μM) for 48 hours. Cytofluorimetric analysis of MCL cell lines mitochondrial potential, as assessed with the mitochondrial specific cationic dye (middle), as described in “Evaluation of mitochondrial membrane potential.” In this representative experiment, treatment with AZ-TAK resulted in mitochondria depolarization in 75% of the cells, compared with control 8% in DMSO-treated cells. Summary of 3 independent experiments demonstrating the effect of AZ-TAK1 on mitochondria membrane polarization (bottom). Each value represents the percentage of cells with depolarized mitochondria. Cells were incubated for 12 hours in the absence (DMSO) or presence of AZ-TAK1 0.5μM.
Activity of AZ-TAK1 in primary lymphoma cells
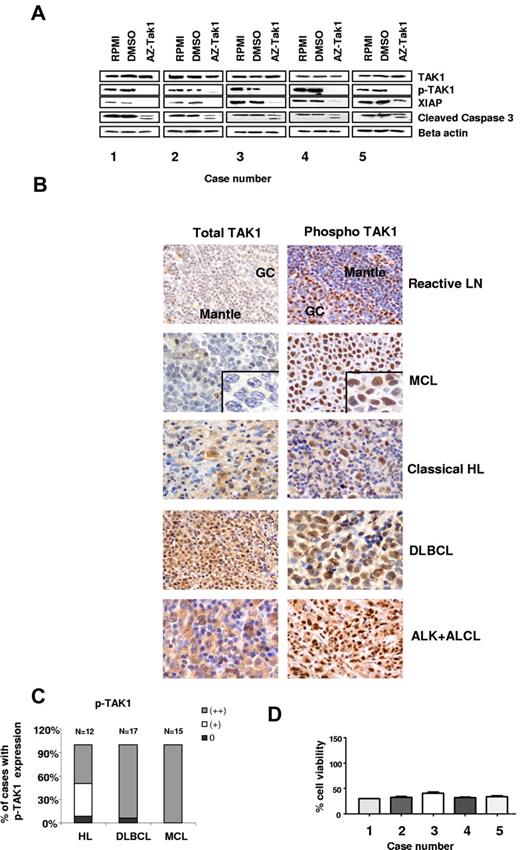
To determine the biologic and molecular effects of AZ-TAK1 in primary lymphoma cells, we examined its activity in 5 MCL specimens isolated from patients with high numbers of circulating lymphoma cells in the peripheral blood. TAK1 and pTAK1 were found to be abundantly expressed by Western blot in primary lymphoma cells (Figure 6A). Using immunohistochemistry methods, we found that TAK1 expression was highly expressed in 6 of 13 (46%) lymph node sections involved with MCL, in 12 of 12 (100%) Hodgkin lymphoma lymph node sections, in 15 of 16 (90%) of diffuse large B-cell lymphoma lymph nodes sections, and in 8 of 8 (100%) of ALK-positive anaplastic large cell lymphoma (Figure 6B-C). Moreover, TAK1 was weakly expressed in 6 additional cases. Similar to what we have observed in the cell line studies, AZ-TAK1 (300nM) inhibited TAK1 phosphorylation, down-regulated XIAP, and cleaved caspase 3 (Figure 6A). These molecular changes were associated induction of cell death in more than 50% of primary lymphoma cells within 24 hours of incubation (Figure 6D)
Effect of AZ-TAK1 on primary lymphoma cells. (A) TAK1 and pTAK1 are abundantly expressed in primary MCL cells. Incubating the cells with 300nM of AZ-TAK1 for 24 hours inhibited TAK1 phosphorylation, down-regulated XIAP, and cleaved caspase 3. (B) TAK1 expression in lymph node sections of primary MCL, classic HL, DLBCL, and ALK (+) ALCL cases. Sections of an infiltrating ductal breast carcinoma and a reactive lymph node (LN) were used as positive and negative controls, respectively. Representative staining of negative case (blastoid type), moderately positive (+), and strongly positive (++) MCL cases are shown. (C) Summary of primary cases expressing pTAK1 by IHC (D) Primary lymphoma cells were incubated with 300nM of AZ-TAK1 for 24 hours before cell viability was determined by the use of the MTS assay. Results are the mean of 3 experiments (± SEM).
Effect of AZ-TAK1 on primary lymphoma cells. (A) TAK1 and pTAK1 are abundantly expressed in primary MCL cells. Incubating the cells with 300nM of AZ-TAK1 for 24 hours inhibited TAK1 phosphorylation, down-regulated XIAP, and cleaved caspase 3. (B) TAK1 expression in lymph node sections of primary MCL, classic HL, DLBCL, and ALK (+) ALCL cases. Sections of an infiltrating ductal breast carcinoma and a reactive lymph node (LN) were used as positive and negative controls, respectively. Representative staining of negative case (blastoid type), moderately positive (+), and strongly positive (++) MCL cases are shown. (C) Summary of primary cases expressing pTAK1 by IHC (D) Primary lymphoma cells were incubated with 300nM of AZ-TAK1 for 24 hours before cell viability was determined by the use of the MTS assay. Results are the mean of 3 experiments (± SEM).
Discussion
In this study, we demonstrated that TAK1 and its active phosphorylated protein are abundantly expressed in a variety of primary and cultured lymphoma cells. We also demonstrated that silencing of TAK1 expression or inhibiting its function in lymphoma cells resulted in inactivation of NF-κB and p38 signaling, in addition to activation of the intrinsic caspase pathway, resulting in lymphoma cell death. Our data suggest that TAK1 may serve as a therapeutic target in lymphoma. Although TAK1 activation mutations have been observed at a very low frequency in lymphoma,26 the underlying mechanisms for TAK1 activation in the majority of lymphomas remain unknown. In these cases, it is possible that TAK1 activation is related to dysregulated cytokine and growth factor stimulation in the lymphoma microenvironment.
In this study, we demonstrated that both cultured and primary MCL cells were sensitive to TAK1 inhibition. Inhibition of TAK1 was associated with the release of cytochrome c from the mitochondria, an important initial step for activating caspase 9. This process was further amplified by the release of SMAC/Diablo and the down-regulation of XIAP in both MCL cell lines and in primary MCL cells.27 XIAP, one of the most potent cellular inhibitors of apoptosis, is known to be overexpressed in a variety of lymphomas.28-30 XIAP activity is inhibited by SMAC/Diablo, which is released from the mitochondria.31 Because inhibiting XIAP function or down-regulating its expression can sensitize cancer cells to chemotherapy, the development of pharmacologic XIAP inhibitors, such as Smac mimetics, is currently under investigation.32 However, Smac mimetics are not sufficient to induce cell death because they work best in combination with other apoptosis-inducing agents.33 In this study, AZ-TAK1 induced apoptosis primarily by releasing cytochrome c, which was amplified by the down-regulation of XIAP and inhibition of its function by releasing SMAC/Diablo. Although, like many other ATP-competitive kinase inhibitors, it cannot be ruled out that AZ-TAK1 exerts some of these effects through inhibition of other kinases, the combined evidence from the siRNA and small molecule data are compelling. Indeed, this triple effect suggests that AZ-TAK1 has potent single-agent activity against lymphoma and may not require the addition of other anticancer agents.
The ability of TAK1 to regulate several survival mechanisms—such as p38, NF-κB, and XIAP—makes it an appealing target for cancer therapy. Nonetheless, pharmacologic inhibition of TAK1 should be explored with caution, given that targeting TAK1 may also result in excessive toxicity to normal tissues. Although we found that AZ-TAK1 was less toxic to PBMCs than to lymphoma cell lines, knockout experiments of TAK1 resulted in massive hepatic and BM toxicity in mice.34 This observation may not preclude the development of TAK1-targeted therapy. For example, although knockout experiments of heat shock protein 90 (HSP90) also proved to be lethal in mice, the authors of recent clinical trials using pharmacologic inhibitors of HSP90 have demonstrated the safety and promising clinical activity of targeting HSP90 in humans.35-37 Furthermore, the potential toxicity to normal tissue can be reduced by exploring novel targeted delivery approaches of TAK1 inhibitors to tumor cells with the use of nanoparticles or antibody-drug conjugates.
In conclusion, our data demonstrate a critical role for TAK1 in promoting MCL cell survival. Future studies should aim at examining the expression pattern and function of TAK1 in other types of lymphoma and should identify factors that are responsible for TAK1 overexpression and activation of TAK1 in lymphoma. These studies can facilitate the development of TAK1-targeted therapy for the treatment of lymphoma.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Virginia M. Mohlere (Department of Quantitive Science Administration, The University of Texas MD Anderson Cancer Center, Houston) for editorial assistance.
This work was supported in part by The University of Texas MD Anderson Cancer Center Support Grant CA016672, the Living Legend Fund, and The Robert and Vicki Smith Fund (to A.Y.).
Authorship
Contribution: D.B. conceived the study, performed experiments, analyzed data, prepared the figures, and wrote the paper; S.P., K.B., and S.S.N. provided valuable reagents, shared data, evaluated the results, and approved the final manuscript; F.V. performed experiments and analyzed data; D.T. and J.S. designed and made the AZ-TAK1 compound and modeled binding mode in Tak1 ATP binding site; and A.Y. conceived the study, helped design experiments, evaluated the results, and approved the final manuscript.
Conflict-of-interest disclosure: S.P., K.B., D.T., and J.S. work for Astra-Zeneca. The remaining authors declare no competing financial interests.
Correspondence: Anas Younes, MD, Department of Lymphoma and Myeloma, Unit 0429, The University of Texas MD Anderson Cancer Center,1515 Holcombe Blvd, Houston, TX 77030; e-mail: ayounes@mdanderson.org.
References
Author notes
S.P. and K.B. contributed equally to this work.

![Figure 3. Identification of the small molecule inhibitor AZ-TAK1. (A) Docked pose of AZ-TAK1 in TAK1 crystal structure. Binding mode of AZ-TAK1 (1-(2-(3-ethyl-5-(5-fluoro-4-(imidazo[1,2-b]pyridazin-3-yl)pyrimidin-2-ylamino)phenoxy)ethyl)piperidin-4-ol) is consistent with in house cocrystal structures of close analogs of AZ-TAK1 (Proteros Biostructures GmbH, unpublished work, August 10, 2008). AZ-TAK1 adopts a U-shaped conformation in TAK1, with the imidazopyridazine heterocycle packing atop C148 and aminopyrimidine making 2 hydrogen bond interactions with the hinge (A81). The 3-ethyl moiety points toward the sugar pocket and the 4-hydroxypiperidine is out in the solvent channel. (B) The ability of AZ-TAK1 to inhibit TAK1 kinase activity was measured in vitro with the use of a complex of recombinant TAK1 and TAB1 and the substrate MKK6kd. Data shown are representative of 3 independent assays. (C) Selectivity of AZ-TAK1 against a panel of 30 kinases.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/2/10.1182_blood-2011-07-369397/4/m_zh89991293810003.jpeg?Expires=1767900383&Signature=pk4M1W7EtUNq0kFZuob8h0PT0xK~hlwM5hO4NTKFXBSs0VFxNeinrNeUvuymMbFbPwD~~WkDSU1b9kCCUxEyjJ~cAMGUCqhDabSmi4-bDHWD4IY291Xy9Q4NLjca3U7tS0SdKQIxhonomRyt0iCZKP321wBhn0RkRczC5FFtz~Sv-CSarzHFwTDB-7ge18ynrmeC9EW5KYd83Cv4BlZN9HbjQMFDyk~CNcfz5qN2fgOJNPfTBAHaWpEUHM30nPu~m0REN~8nqusSbhY8p69yIOT0xL4D8~Ih9-lqYIO47Wlf6kI0tOB87GwIvkQw9mXgcdZTjE5e81~JvQhXZRpOFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)