Abstract
In red cell development, the differentiation program directed by the transcriptional regulator GATA1 requires signaling by the cytokine erythropoietin, but the mechanistic basis for this signaling requirement has remained unknown. Here we show that erythropoietin regulates GATA1 through protein kinase D activation, promoting histone deacetylase 5 (HDAC5) dissociation from GATA1, and subsequent GATA1 acetylation. Mice deficient for HDAC5 show resistance to anemic challenge and altered marrow responsiveness to erythropoietin injections. In ex vivo studies, HDAC5−/− progenitors display enhanced entry into and passage through the erythroid lineage, as well as evidence of erythropoietin–independent differentiation. These results reveal a molecular pathway that contributes to cytokine regulation of hematopoietic differentiation and offer a potential mechanism for fine tuning of lineage-restricted transcription factors by lineage-specific cytokines.
Introduction
Erythropoiesis provides a well-defined paradigm for dissecting the roles of extrinsic and intrinsic influences in hematopoietic differentiation. Erythropoietin signaling via its receptor, EpoR, and transcriptional control by the master regulator GATA1 are both required for proper red cell development in vitro and in vivo.1 The cooperative relationship between erythropoietin signal transduction and GATA1 programming of erythropoiesis was first established by Gregory et al using progenitors expressing a conditional GATA1 mutant.2 In the presence of erythropoietin, GATA1 activation potently induced erythroid differentiation, but in the absence of erythropoietin GATA1 activation caused extensive cell death with minimal erythroid differentiation. The molecular basis for this cooperative interaction has been intensively studied as it potentially elucidates a mechanism for extrinsic signaling exerting regulatory control over “intrinsic” transcriptional machinery.
Candidate pathways for erythropoietin regulation of differentiation have involved either GATA1 phosphorylation by AKT or GATA1 stabilization by HSP70.3-5 With regard to the former pathway, 2 groups identified serine 310 on GATA1 as a target of AKT phosphorylation downstream of erythropoietin activation of PI3K.3,4 Intriguingly, transfection of constitutively active AKT eliminated requirements for either erythropoietin or JAK2 signaling in erythroid differentiation.4,6 However, the GATA1 S310A mutant retained the capacity to program erythropoiesis in vitro,3,4 and knock-in mice expressing GATA1 S310A showed no abnormalities in steady-state or stress erythropoiesis.7 Therefore, the significance of erythropoietin–induced GATA1 phosphorylation remains to be determined. In the latter pathway, human erythroid progenitors subjected to erythropoietin-starvation displayed HSP70 dissociation from GATA1 and nuclear export, exposing GATA1 to caspase–mediated cleavage.5 Whether subcellular localization of HSP70 reflects a specific erythropoietin signaling mechanism or simply levels of cellular stress is unclear. In addition, an in vivo role of HSP70 in erythropoiesis remains unestablished. Thus, how or even whether erythropoietin can “instruct” erythropoiesis through regulation of GATA-1 function has not been resolved.
In the present work, erythropoietin is revealed to activate a protein kinase D-class IIa histone deacetylase (PKD-HDAC) signaling pathway previously implicated in programming differentiation of muscle lineages.8,9 The class IIa HDACs compose HDAC4, HDAC5, HDAC7, and HDAC9. Prior studies in murine erythroleukemia (MEL) cells have demonstrated an interaction of endogenous HDAC5 and GATA1, with disruption of this interaction associated with chemical induction of differentiation.10 We now show that erythropoietin signaling promotes the dissociation of HDAC5 from GATA1 and causes GATA1 acetylation, a modification possibly involved in transcriptional programming of erythropoiesis.11 Interference with PKD signaling impairs erythroid differentiation in the presence of erythropoietin, whereas knockdown of HDAC5 heightens erythropoietin responsiveness. Mice lacking HDAC5 show resistance to anemic challenge, enhanced progenitor entry into the erythroid lineage, accelerated erythroid maturation in response to erythropoietin, and a capacity for erythropoietin–independent erythroid maturation. These findings therefore delineate a mechanism for the regulation of GATA1 by erythropoietin signaling and offer a paradigm in which a lineage specific hematopoietic cytokine may directly tune the activity of a lineage-selective transcriptional master regulator.
Methods
Cell culture
G1ER cells, as previously described,2 were maintained in IMDM with 15% FBS, 50 ng/mL murine SCF, and 2 U/mL recombinant human erythropoietin (G1ER maintenance medium). For induction of GATA1–mediated differentiation, the cells were treated 48 hours with 10nM estradiol (Sigma-Aldrich). For kinase inhibition studies, G1ER cells received 1μM Gö6976 or Gö6983 (EMD) for the duration of differentiation induction. For analysis of erythropoietin activation of PKD phosphorylation, cells were cultured overnight in G1ER maintenance medium lacking erythropoietin followed by treatment with 2 U/mL erythropoietin for the indicated durations. For HDAC inhibition studies, the cells were treated with 2μM suberoylanilide hydroxamic acid (SAHA; Indofine Chemical Co) throughout differentiation induction.
Purified primary human CD34+ hematopoietic progenitors were cultured in serum-free erythroid unilineage media with 100% or 15% transferrin saturation as described.12 For kinase inhibition, Gö6976 or Gö6983 was included in the medium at doses of 0.5-2μM. For analysis of erythropoietin activation of PKD phosphorylation, cells from day 3 erythroid culture underwent 3 hours of cytokine deprivation followed by stimulation with 4.5 U/mL erythropoietin. For culture of murine marrow progenitors, FACS-sorted CD71Bright Ter119− cells from wild-type (WT) and HDAC5−/− animals were seeded in G1ER maintenance medium with or without 2 U/mL erythropoietin in 6-well plates for the indicated durations.
shRNA knockdowns
For lentiviral shRNA knockdown of PKD3, constructs in the pLKO.1 vector (Open Biosystems catalog RMM4534-NM_029239, Thermo Scientific) were packaged by triple transfection of 293T cells as described13 ; G1ER cells underwent spinoculation in viral supernatants, followed by selection in puromycin and screening for knockdown by immunoblot. shRNA constructs screened for knockdowns of murine HDAC5 and HDAC4 in G1ER cells came from the Open Biosystem sets RMM4532-NM_001077696 and RMM4534-NM_207225 (Thermo Scientific). Knockdown of protein kinase Cα (PKC-α) in primary human CD34+ cells was carried out as previously described.12
Immunoblots, immunoprecipitations, and subcellular fractionation
The following antibodies were used for immunoblot analysis of whole cell lysates: rabbit anti-PKD3 isoform-specific (Anaspec), rabbit anti-PKD recognizing all isoforms (Cell Signaling), rabbit anti-PKD phosphorylated on S744/748 (Cell Signaling), rabbit anti-PKD1 isoform-specific (Acris), rabbit anti-PKD2 isoform-specific (Cell Signaling), rabbit anti-HDAC4 (Cell Signaling), rabbit anti-HDAC5 (Cell Signaling), rabbit anti-HDAC7 (Cell Signaling), rabbit anti-HDAC9 (Santa Cruz Biotechnology), rabbit antibody specific for phosphorylated class IIa HDACs (HDAC5 on S498; HDAC4 on S632; Cell Signaling), rabbit anti–PKC-α (Cell Signaling), and mouse antitubulin (Sigma-Aldrich). Detailed descriptions of the methods for analyzing GATA1-HDAC5 interactions by immunoprecipitation are provided as supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To analyze protein lysine acetylation, immunoprecipitations were performed as we have described.14 Briefly, cells cultured as for the GATA1 immunoprecipitations were subjected to whole cell extraction step, followed by immunoprecipitation overnight at 4°C with either rabbit anti–acetyl-lysine (AB3879, Millipore) or normal rabbit IgG. Immune complexes underwent capture, washing, and immunoblot analysis with rat anti-GATA1 (N1), mouse anti-p300 (NM11), and goat anti-SCL/TAL (all from Santa Cruz Biotechnology). The NE-PER nuclear and cytosolic extraction kit (Thermo Scientific) was used for subcellular fractionation, supplementing the extraction buffers with the Mini-EDTA-free Protease Inhibitor Cocktail-Complete (Roche Diagnostics). Monoclonal rabbit anti–poly(ADP-ribose) polymerase (Cell Signaling) and polyclonal rabbit anti–lactate dehydrogenase recognizing isoforms A-C (Santa Cruz Biotechnology) were used on immunoblots to control for purity of nuclear and cytosolic fractions, respectively, as well as for lane loading controls.
Animal model
HDAC5−/− mice have been previously described.15 The mice were on a mixed C57BL/6–129sv genetic background and were analyzed at 6-8 weeks of age. Blood obtained by retro-orbital phlebotomy was analyzed for complete blood count values on Hemavet 850 and 950FS analyzers (Drew Scientific). For anemia induction, the animals received 2 intraperitoneal injections, on days −1 and 0, of phenylhydrazine-HCl in PBS, each at a dose of 60 mg/kg body weight. For erythropoietin treatment, animals received weekly intraperitoneal injections of darbepoetin alfa (Amgen) at 25 μg/kg per injection, for a total of 3 injections.
Flow cytometry, hemoglobin staining, colony forming assays, and histology
A FACSCalibur instrument (BD Biosciences), and FlowJo software (Version 8.6.3; TreeStar) were used for flow cyometry. Fluorochrome-conjugated antibodies were purchased from BD Biosciences PharMingen. Costaining for glycophorin A and CD41 on primary human progenitors was performed as previously described.12 Erythroid maturation of murine marrow cells was determined by costaining for Ter119 and CD71 as described.16 Benzidine staining for hemoglobin was performed as described.2 To quantitate colony-forming units, 2 × 104 marrow cells were plated in 1 mL Methocult M3434 (Stem Cell Technologies) in a 35-mm plate. For each animal, duplicate cultures were performed, with CFU-GM counted on day 5; BFU-E and CFU-GEMM were counted on day 7. To detect splenic iron deposition, sections from formalin-fixed, paraffin-embedded tissue underwent staining with potassium ferrocyanide HCl according to the method of Gomori, with Nuclear Fast Red counterstain.17
Statistical analysis
Statstical comparisons between 2 groups used Student t test (2-tailed, unpaired), and comparisons among > 2 groups used ANOVA.
Results
Involvement of protein kinase D in erythropoietic regulation
PKC-α initially was identified as a positive signaling element in erythropoietin induction of erythroid differentiation in human progenitors.18 Subsequent work, however, has provided evidence for an inhibitory function for PKC-α/β in this system.12 Notably, erythropoietin deprivation, rather than stimulation, promoted activation of PKC-α/β; by contrast, the activation status of PKC-μ, also known as PKD, correlated directly with erythropoietin levels.12 Furthermore, shRNA knockdown of PKC-α did not impair erythroid differentiation in iron–deprived progenitors12 and actually enhanced erythroid differentiation of iron replete progenitors (supplemental Figure 1). We therefore addressed whether PKD, rather than PKC-α, may be promoting erythropoietin–mediated erythroid differentiation.
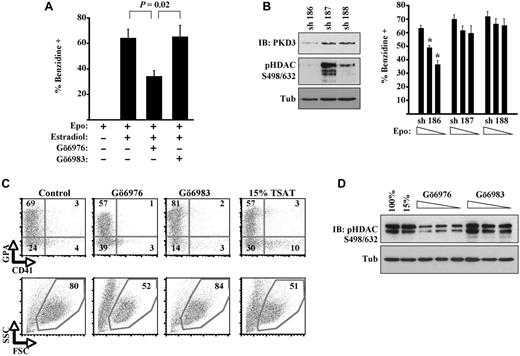
Initial experiments examining the role of PKD in erythroid differentiation used murine erythroid progenitors expressing a conditional GATA1 mutant (G1ER cells). Activation of the GATA1-ER fusion by estradiol treatment has been shown to induce rapid and efficient erythroid differentiation in the presence of erythropoietin.2 In our experiments, treatment of the cells with Gö6976, a potent inhibitor of both PKD and classic PKC isoenzymes,19 significantly inhibited erythroid differentiation, as reflected by diminished percentages of hemoglobinized cell staining with benzidine (Figure 1A). By contrast, treatment of the cells with Gö6983, an equally potent inhibitor of classic PKC isoenzymes but weak inhibitor of PKD,19 caused no inhibition of erythroid differentiation. These results suggest that PKD, rather than PKC-α, contributes to erythropoietin signaling supporting differentiation.
Implication of PKD in erythropoiesis. (A) G1ER proerythroblasts underwent differentiation induction with erythropoietin (Epo), estradiol, and kinase inhibitors; percentage hemoglobinized cells was assessed by benzidine staining. Data are mean ± SEM for 3 independent experiments. (B) G1ER cells transduced with shRNA constructs targeting PKD3 were assessed for PKD3 expression, class IIa HDAC phosphorylation, and erythroid differentiation. sh 186 provided strong knockdown of PKD3 expression; sh 187 provided no knockdown; and sh 188 provided weak knockdown. Left: Immunoblot (IB) of transduced cells (8% gel). Right: Differentiation induction with estradiol and erythropoietin (0.5, 0.1, or 0.05 U/mL), showing mean ± SEM for 3 experiments. *P < .05 for sh 186 vs controls at corresponding Epo doses. (C) Human primary progenitors cultured 5 days in erythroid medium with 100% or 15% transferrin saturation. Kinase inhibitors were included at 2μM in medium with 100% transferrin saturation. Flow cytometry for the erythroid marker glycophorin A (GPA) and the megakaryocyte marker CD41, with gating on viable fractions by forward (FSC) and side scatter (SSC). See also supplemental Figure 1. (D) Class IIa HDAC phosphorylation in human progenitors cultured 5 days in erythroid medium with 100% or 15% transferrin saturation (TSAT) or with kinase inhibitors (0.5, 1.0, and 2.0μM, all with 100% transferrin saturation). IB of whole cell lysates (12% gel).
Implication of PKD in erythropoiesis. (A) G1ER proerythroblasts underwent differentiation induction with erythropoietin (Epo), estradiol, and kinase inhibitors; percentage hemoglobinized cells was assessed by benzidine staining. Data are mean ± SEM for 3 independent experiments. (B) G1ER cells transduced with shRNA constructs targeting PKD3 were assessed for PKD3 expression, class IIa HDAC phosphorylation, and erythroid differentiation. sh 186 provided strong knockdown of PKD3 expression; sh 187 provided no knockdown; and sh 188 provided weak knockdown. Left: Immunoblot (IB) of transduced cells (8% gel). Right: Differentiation induction with estradiol and erythropoietin (0.5, 0.1, or 0.05 U/mL), showing mean ± SEM for 3 experiments. *P < .05 for sh 186 vs controls at corresponding Epo doses. (C) Human primary progenitors cultured 5 days in erythroid medium with 100% or 15% transferrin saturation. Kinase inhibitors were included at 2μM in medium with 100% transferrin saturation. Flow cytometry for the erythroid marker glycophorin A (GPA) and the megakaryocyte marker CD41, with gating on viable fractions by forward (FSC) and side scatter (SSC). See also supplemental Figure 1. (D) Class IIa HDAC phosphorylation in human progenitors cultured 5 days in erythroid medium with 100% or 15% transferrin saturation (TSAT) or with kinase inhibitors (0.5, 1.0, and 2.0μM, all with 100% transferrin saturation). IB of whole cell lysates (12% gel).
To determine more specifically the contributions of PKD signaling in this experimental system, G1ER cells underwent shRNA knockdown of PKD3, one of 2 isoforms detectable in erythroid cells (supplemental Figure 2A). Knocking down PKD3 diminished steady-state phosphorylation of class IIa HDAC4 and HDAC5 (Figure 1B), confirming a signaling role for this isoform. Impairment of G1ER differentiation because of this knockdown became increasingly evident as erythropoietin levels in the medium were decreased (Figure 1B). This finding supports a contribution by PKD3 to erythroid differentiation but also suggests that a strict requirement might be bypassed with supraphysiologic levels of erythropoietin, possibly by driving activation of PKD2 or related kinases within the CAMK group.20
The participation of PKD in erythroid differentiation was also assessed in primary human hematopoietic progenitors cultured in unilineage medium as described.12 In this system, the PKD/PKC antagonist Gö6976 inhibited both differentiation and viability, whereas the more selective PKC antagonist Gö6983 actually enhanced differentiation and viability (Figure 1C). As previously published,12 iron deprivation consisting of 15% transferrin saturation also impaired erythroid differentiation and viability (Figure 1C). With regard to signaling, Gö6976 treatment diminished class IIa HDAC phosphorylation in a dose–dependent manner, confirming the presence of PKD-HDAC signaling during normal erythroid differentiation (Figure 1D). This signaling was unaffected by iron deprivation (15%) and was enhanced by selective PKC inhibition. These results thus implicate PKD-HDAC signaling in erythroid differentiation of primary human progenitors.
Erythropoietin activation of PKD in erythoid progenitors
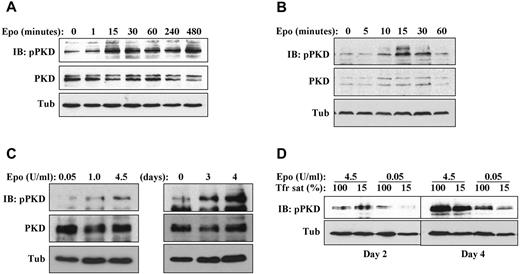
Assessment of PKD activation was conducted using a phospho-specific antibody to serines 744 and 748, an antibody that has been validated by Rybin et al as both sensitive and specific for detecting PKD activation.21 This antibody typically detects multiple species because of: (1) its recognition of multiple PKD isoforms, (2) the various mobilities of PKD induced by multisite phosphorylation, and (3) complex patterns of proteolysis induced by calpain and caspase.21-23 To determine whether PKD activation occurred as a consequence of erythropoietin signaling, G1ER cells maintained overnight in SCF alone were stimulated with erythropoietin followed by time-course analysis of PKD phosphorylation. In this setting, erythropoietin up-regulated phosphorylation of full-length (∼ 120 kDa) PKD, initiating at 15 minutes and continuing for 8 hours (Figure 2A; supplemental Figure 2C). In cytokine–deprived human primary progenitors, erythropoietin treatment also activated full-length PKD phosphorylation, initially observed at 10-15 minutes and sustained for 30 minutes (Figure 2B; supplemental Figure 2B). Multiple independent repeat experiments analyzed by scanning densitometry confirmed the up-regulation of PKD phosphorylation by erythropoietin in primary human progenitors (supplemental Figure 2B,D) and in G1ER cells (supplemental Figure 2E). Under continuous exposure to erythropoietin, the degree of PKD phosphorylation varied according to erythropoietin dosage and duration of exposure (Figure 2C-D). Iron deprivation, despite having an inhibitory effect on erythroid differentiation, did not impair PKD activation under conditions of high erythropoietin (Figure 2D), in agreement with previously published data.12
Erythropoietin induction of PKD phosphorylation. (A) Erythropoietin–deprived G1ER cells were stimulated with erythropoietin, followed by immunoblotting for phospho- and total PKD. (B) Cytokine-starved human erythroid progenitors were stimulated with erythropoietin and analyzed as in panel A. (C) Impact of erythropoietin dosage and duration on steady-state PKD phosphorylation. Left: Human progenitors cultured 4 days with the indicated doses of erythropoietin. Right: Human progenitors cultured for the indicated durations with 4.5 U/mL erythropoietin. (D) Impact of iron availability on steady-state PKD phosphorylation. Human progenitors were cultured in erythroid medium under either iron replete (100% transferrin saturation) or iron restricted (15% transferrin saturation) conditions.
Erythropoietin induction of PKD phosphorylation. (A) Erythropoietin–deprived G1ER cells were stimulated with erythropoietin, followed by immunoblotting for phospho- and total PKD. (B) Cytokine-starved human erythroid progenitors were stimulated with erythropoietin and analyzed as in panel A. (C) Impact of erythropoietin dosage and duration on steady-state PKD phosphorylation. Left: Human progenitors cultured 4 days with the indicated doses of erythropoietin. Right: Human progenitors cultured for the indicated durations with 4.5 U/mL erythropoietin. (D) Impact of iron availability on steady-state PKD phosphorylation. Human progenitors were cultured in erythroid medium under either iron replete (100% transferrin saturation) or iron restricted (15% transferrin saturation) conditions.
Involvement of HDAC5 in erythropoietin cooperation with GATA1
Class I and II HDACs are known to interact with the hematopoietic GATA factors, GATA2 and GATA1.10,24 We therefore addressed whether erythropoietin signaling might influence GATA1 function through HDAC inhibition. In initial experiments, G1ER cells underwent GATA1 activation in medium lacking erythropoietin but containing the pan-HDAC inhibitor SAHA. As shown in Figure 3A-B, SAHA permitted erythropoietin–independent differentiation and survival of the estradiol–induced G1ER cells, although the rescue of erythroid differentiation by SAHA, while significant, was incomplete. These results suggest that erythropoietin signaling may cooperate with GATA1 activation through HDAC inhibition, extending previous observations in which HDAC inhibitors promoted erythropoietin–independent erythroid maturation of human progenitors.25,26
Functional implication of HDAC5 as a target of erythropoietin signaling. (A-B) HDAC inhibition partially substitutes for erythropoietin signaling during GATA1–induced erythroid differentiation. G1ER cells induced with estradiol ± erythropoietin (Epo) and ± SAHA were assessed for differentiation in panel A and viability in panel B. Data are mean ± SEM for 3 experiments. (C-E) G1ER cells transduced with shRNA constructs targeting HDAC5 were assessed for HDAC5 and HDAC4 expression, erythroid differentiation, and viability. (C) Immunoblot (IB) of whole cell lysates. sh 253 provided strong knockdown of HDAC5, and sh 252 provided no knockdown of HDAC5. (D) Differentiation of cells induced with estradiol and erythropoietin (0.5, 0.1, or 0.05 U/mL). *P = .01 for sh 253 vs control at corresponding Epo dose in 3 experiments. (E) Viability of cells induced with estradiol and erythropoietin. *P = .03. See also supplemental Figure 3 for HDAC4 knockdown data. (F-G) Distinct subcellular localization patterns of HDAC5 and HDAC4 in erythroid progenitors. (F) G1ER cells stimulated with erythropoietin (Epo) for the indicated durations were subjected to subcellular fractionation followed by immunoblotting for HDACs. Poly(ADP-ribose) polymerase and lactate dehydrogenase served as loading controls for nuclear and cytosolic fractions, respectively. (G) Human CD34+ progenitors grown in erythroid medium for the indicated durations were analyzed as in panel F.
Functional implication of HDAC5 as a target of erythropoietin signaling. (A-B) HDAC inhibition partially substitutes for erythropoietin signaling during GATA1–induced erythroid differentiation. G1ER cells induced with estradiol ± erythropoietin (Epo) and ± SAHA were assessed for differentiation in panel A and viability in panel B. Data are mean ± SEM for 3 experiments. (C-E) G1ER cells transduced with shRNA constructs targeting HDAC5 were assessed for HDAC5 and HDAC4 expression, erythroid differentiation, and viability. (C) Immunoblot (IB) of whole cell lysates. sh 253 provided strong knockdown of HDAC5, and sh 252 provided no knockdown of HDAC5. (D) Differentiation of cells induced with estradiol and erythropoietin (0.5, 0.1, or 0.05 U/mL). *P = .01 for sh 253 vs control at corresponding Epo dose in 3 experiments. (E) Viability of cells induced with estradiol and erythropoietin. *P = .03. See also supplemental Figure 3 for HDAC4 knockdown data. (F-G) Distinct subcellular localization patterns of HDAC5 and HDAC4 in erythroid progenitors. (F) G1ER cells stimulated with erythropoietin (Epo) for the indicated durations were subjected to subcellular fractionation followed by immunoblotting for HDACs. Poly(ADP-ribose) polymerase and lactate dehydrogenase served as loading controls for nuclear and cytosolic fractions, respectively. (G) Human CD34+ progenitors grown in erythroid medium for the indicated durations were analyzed as in panel F.
PKD directly phosphorylates class IIa HDACs on conserved serine residues; the phosphorylated HDACs then undergo dissociation from transcriptional complexes, promoting derepression of target genes.8,9 Class IIa HDACs expressed in G1ER cells and human erythroid progenitors included HDAC4 and HDAC5, the former cytosolic and the latter nuclear (Figure 3F-G). No significant expression of HDAC7 or HDAC9 was identified in human erythroid progenitors (supplemental Figure 3A). Erythropoietin stimulation caused a minor, delayed down-regulation of HDAC5 but no major shift in subcellular localization. Notably, shRNA knockdown of HDAC5 significantly enhanced erythropoietin sensitivity of G1ER cells activated with estradiol (Figure 3C-E). Thus, cells deficient in HDAC5 retained full capacity for differentiation and viability in the presence of limiting doses of erythropoietin. In similar assays, knocking down HDAC4 did not significantly enhance erythropoietin responsiveness (supplemental Figure 3A-C).
Erythropoietin signaling promotes HDAC5 dissociation from and acetylation of GATA1
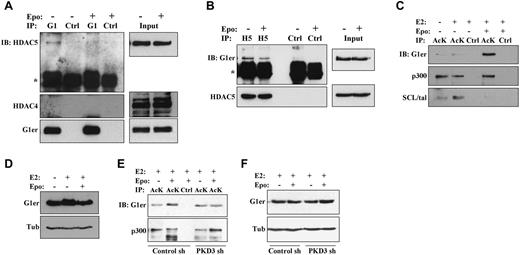
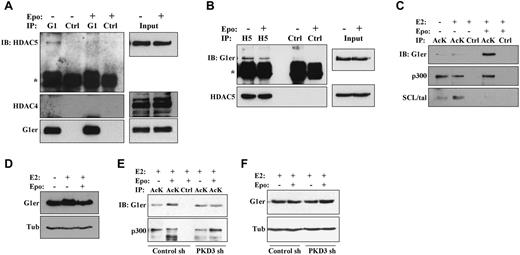
Chemical induction of murine erythroleukemic cell differentiation has been associated with disruption of endogenous HDAC5-GATA1 protein complexes.10 To determine the relevance of this finding to erythropoietin signaling in nontransformed erythroid progenitors, G1ER cells without or with erythropoietin stimulation underwent immunoprecipitation of GATA1 followed by immunoblotting for class IIa HDACs. In the absence of erythropoietin, HDAC5 but not HDAC4 coprecipitated with GATA1 (Figure 4A). Importantly, erythropoietin stimulation of cells for 2 hours diminished coprecipitation of HDAC5 with GATA1. Contribution of PKD to this dissociation was supported by immunoprecipitation studies in PKD3 knockdown cells, which showed increased GATA1 binding to HDAC5 in the presence of erythropoietin (supplemental Figure 4A). Because of the poor immunoprecipitation efficiency of available antibodies to mouse HDAC5, reverse immunoprecipitation studies were conducted using anti–human HDAC5 on G1ER cells transduced with a human HDAC5 cDNA (supplemental Methods). In these cells, erythropoietin treatment was associated with decreased coprecipitation of GATA1 with HDAC5 (Figure 4B). Quantitative scanning densitometry, with normalization for antibody immunoprecipitation efficiencies of target antigen, showed that erythropoietin treatment diminished the HDAC5-GATA1 interaction by 75% in Figure 4A and by 65% in Figure 4B.
Erythropoietin promotes dissociation of GATA1-HDAC5 complexes and acetylation of GATA1. (A) Analysis of GATA1 interaction with HDAC5 by GATA1 immunoprecipitation (IP). Extracts from G1ER cells ± erythropoietin (Epo) stimulation underwent IP with anti-GATA1 (G1) or control (Ctrl) antibodies followed by immunoblotting (IB) for HDAC5, HDAC4, and GATA1. “G1er” refers to the position of the GATA1-ER fusion. *Immunoglobulin heavy chain. See also supplemental Figure 4A. (B) Analysis of HDAC5 interaction with GATA1 by HDAC5 immunoprecipitation (IP). Extracts from G1ER cells expressing human HDAC5 ± Epo treatment underwent IP with anti–human-HDAC5 (H5) or control (Ctrl) antibodies followed by immunoblotting (IB) for GATA1 and HDAC5. “G1er” refers to the position of the GATA1-ER fusion. *Immunoglobulin heavy chain. (C-D) Analysis of GATA1 acetylation. (C) Extracts from G1ER cells ± erythropoietin stimulation underwent immunoprecipitation (IP) with anti–acetyl-lysine (AcK) or control (Ctrl) antibodies followed by immunoblotting. (D) Input levels of GATA1. (E-F) Contribution of PKD3 to erythropoietin–induced GATA1 acetylation. G1ER cells transduced with the sh 186 (PKD3 sh) and sh 187 (Control sh) constructs (see Figure 1B) were analyzed for GATA1 acetylation as in panels B and C. See also supplemental Figure 4.
Erythropoietin promotes dissociation of GATA1-HDAC5 complexes and acetylation of GATA1. (A) Analysis of GATA1 interaction with HDAC5 by GATA1 immunoprecipitation (IP). Extracts from G1ER cells ± erythropoietin (Epo) stimulation underwent IP with anti-GATA1 (G1) or control (Ctrl) antibodies followed by immunoblotting (IB) for HDAC5, HDAC4, and GATA1. “G1er” refers to the position of the GATA1-ER fusion. *Immunoglobulin heavy chain. See also supplemental Figure 4A. (B) Analysis of HDAC5 interaction with GATA1 by HDAC5 immunoprecipitation (IP). Extracts from G1ER cells expressing human HDAC5 ± Epo treatment underwent IP with anti–human-HDAC5 (H5) or control (Ctrl) antibodies followed by immunoblotting (IB) for GATA1 and HDAC5. “G1er” refers to the position of the GATA1-ER fusion. *Immunoglobulin heavy chain. (C-D) Analysis of GATA1 acetylation. (C) Extracts from G1ER cells ± erythropoietin stimulation underwent immunoprecipitation (IP) with anti–acetyl-lysine (AcK) or control (Ctrl) antibodies followed by immunoblotting. (D) Input levels of GATA1. (E-F) Contribution of PKD3 to erythropoietin–induced GATA1 acetylation. G1ER cells transduced with the sh 186 (PKD3 sh) and sh 187 (Control sh) constructs (see Figure 1B) were analyzed for GATA1 acetylation as in panels B and C. See also supplemental Figure 4.
Acetylation of GATA1 during erythropoiesis may contribute to its recruitment to target genes and subsequent programming of differentiation.11,27,28 The effect of erythropoietin stimulation on GATA1 acetylation was therefore examined by subjecting G1ER cells to immunoprecipitation with an antibody directed to acetyl-lysine followed by immunoblot analysis. Stimulation of the cells with erythropoietin for 2 hours strongly enhanced GATA1 acetylation but had no effect on p300 acetylation (Figure 4C-D). SCL/tal, an additional erythroid transcription factor regulated by P/CAF acetylation,29 showed decreased acetylation in response to erythropoietin treatment. The erythropoietin–induced increase in GATA1 acetylation was abrogated by PKD3 knockdown (Figure 4E-F; supplemental Figure 4B-C). Therefore, erythropoietin–induced activation of PKD contributes to dissociation of HDAC5 from GATA1 and GATA1 acetylation.
Altered marrow progenitor composition and response to anemia in mice lacking HDAC5
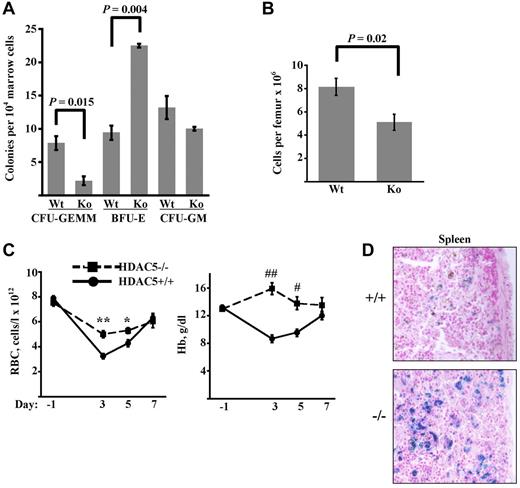
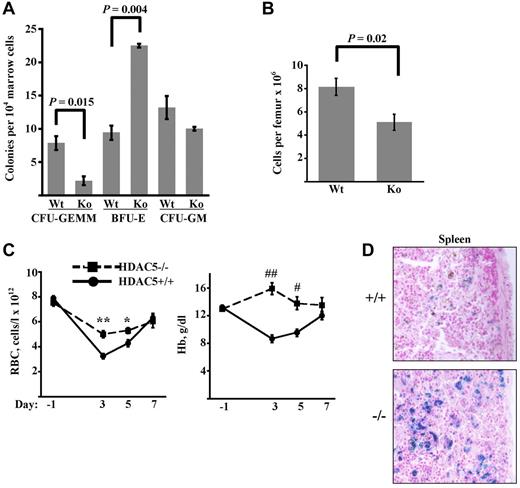
Mice lacking HDAC5 are viable and fertile but display exaggerated cardiac hypertrophy under hemodynamic stress.9,15 To determine the influence of HDAC5 on steady-state marrow progenitor composition, colony-forming assays were conducted on WT and HDAC5−/− adult mice (Figure 5A). The overall marrow cellularity was slightly but significantly diminished in the HDAC5−/− mice (Figure 5B). More strikingly, HDAC5−/− mice displayed a 4-fold decrease in the frequency of multipotent CFU-GEMM combined with a 2-fold increase in erythroid committed BFU-E (Figure 5A). Frequency of myeloid-committed CFU-GM did not differ between strains. Comparison of steady-state peripheral blood parameters revealed a significant (∼ 35%) increase in red cell mean corpuscular volume in HDAC5−/− animals, contributing to increased hematocrit and decreased mean corpuscular hemoglobin concentration (supplemental Table 1). Microscopic examination of peripheral blood smears confirmed in HDAC5−/− animals enlargement of erythrocytes and identified occasional target cells (supplemental Figure 5A). No differences in red cell numbers (RBC) or overall hemoglobin levels (Hb) were seen.
Increased marrow BFU-E frequency and enhanced responsiveness to anemic challenge in HDAC5−/− mice. (A) Colony-forming progenitor frequencies in marrows of adult WT and HDAC5−/− (KO) mice. Data are mean ± SEM (n = 3/group). (B) Marrow cellularity in adult WT and HDAC5−/− mice (n = 4/group). (C) RBC and Hb levels in animals before and after anemia induction. HDAC5+/+ and HDAC5−/− mice received PHZ on days −1 and 0. Data are mean ± SEM (n = 7/group). **P < .001. *P = .017. ##P < .0001. #P = 0.003. (D) Staining for iron deposition in HDAC5+/+ and HDAC5−/− spleens on day 13 after anemia induction. Shown are light microscope images (original magnification × 200) representative of findings in all animals studied (3/group). Images were acquired using an Olympus BX51 microscope equipped with an Olympus DP70 digital camera. The objective lens consisted of Uplan Fl 20×/0.75 NA. Image acquisition and processing were used: Adobe Photoshop, CS3/10.0 and CS2/9.0, respectively.
Increased marrow BFU-E frequency and enhanced responsiveness to anemic challenge in HDAC5−/− mice. (A) Colony-forming progenitor frequencies in marrows of adult WT and HDAC5−/− (KO) mice. Data are mean ± SEM (n = 3/group). (B) Marrow cellularity in adult WT and HDAC5−/− mice (n = 4/group). (C) RBC and Hb levels in animals before and after anemia induction. HDAC5+/+ and HDAC5−/− mice received PHZ on days −1 and 0. Data are mean ± SEM (n = 7/group). **P < .001. *P = .017. ##P < .0001. #P = 0.003. (D) Staining for iron deposition in HDAC5+/+ and HDAC5−/− spleens on day 13 after anemia induction. Shown are light microscope images (original magnification × 200) representative of findings in all animals studied (3/group). Images were acquired using an Olympus BX51 microscope equipped with an Olympus DP70 digital camera. The objective lens consisted of Uplan Fl 20×/0.75 NA. Image acquisition and processing were used: Adobe Photoshop, CS3/10.0 and CS2/9.0, respectively.
Response to anemic challenge was assessed by administration of the hemolytic agent phenylhydrazine (PHZ). PHZ-treated HDAC5−/− mice developed an unusually mild anemia, with nadir RBC values 56% above those of WT mice (Figure 5C). Remarkably, Hb levels in HDAC5−/− mice, in contrast to those in WT animals, showed no decline at any of the time points assessed. A potential explanation for the blunted anemic response in HDAC5−/− mice is diminished hemolysis caused by PHZ. The magnitude of this hemolysis is reflected by iron deposition in the spleen, where the damaged red cells undergo phagocytosis. Staining for iron showed increased iron deposition in HDAC5−/− spleens, consistent with increased red cell turnover (Figure 5D). Measurement of endogenous serum erythropoietin levels showed no significant differences between WT and HDAC5−/− mice before PHZ or at the RBC nadir, although HDAC5−/− mice displayed a trend toward lower erythropoietin levels at the RBC nadir (supplemental Figure 5B). Similarly, pre-PHZ and nadir reticulocyte counts did not significantly differ between the 2 groups (supplemental Figure 5C).
Altered marrow response to exogenous erythropoietin in mice lacking HDAC5
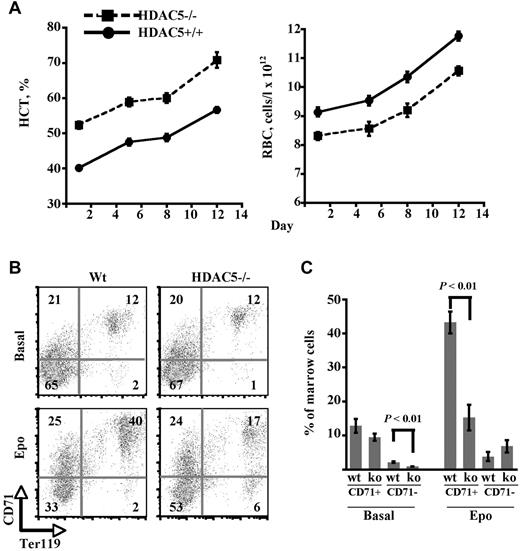
The in vitro data implicate HDAC5 as a target of erythropoietin signaling and show that knockdown of HDAC5 renders erythroblasts more responsive to limiting doses of erythropoietin (Figures 3 and 4). To determine the influence of HDAC5 on responsiveness to exogenous erythropoietin in vivo, WT and HDAC5−/− mice received weekly intraperitoneal injections of the long-acting erythropoietin formulation, darbepoetin alfa. In the peripheral circulation, WT and HDAC5−/− animals responded to this treatment with similar increases in hematocrit and RBC (Figure 6A). Spleen findings were also similar in both groups (ie, no enlargement and minor increases in splenic erythropoiesis in response to the doses of darbepoetin provided; not shown). By contrast, the marrow responses markedly differed between the 2 strains (Figure 6B-C). Erythropoietin-treated WT mice, as previously described,30 selectively expanded the compartment of CD71-positive erythroid progenitors, with a 3- to 4-fold increase in CD71+ Ter119+ cells (Figure 6B-C). Strikingly, HDAC5−/− mice treated with erythropoietin displayed no change in the proportion of CD71+ erythroid progenitors within the marrow. These results suggest that the marrow response to erythropoietin in HDAC5−/− mice, unlike that of WT mice, does not involve expansion of a proerythroblastic pool as described by Dev et al.30
Altered marrow response to exogenous erythropoietin in HDAC5−/− mice. (A) Hematocrit (HCT) and RBC in adult WT and HDAC5−/− animals receiving weekly injections of the long-acting agent darbepoetin alfa. N = 6 animals/group. (B) Erythroid maturation in marrows of WT and HDAC5−/− mice after either no treatment (basal) or 3 injections of darbepoetin alfa (Epo). Flow cytometry for expression of CD71 and Ter119, with percentages shown within indicated quadrants. (C) Comparison of percentages of the less mature Ter119+ CD71+ progenitors and of the more mature Ter119+ CD71− progenitors. Composite of data from panel B analyzing marrows from WT or HDAC5−/− (KO) mice either untreated (basal) or darbepoetin-treated (mean ± SEM; n = 3/group).
Altered marrow response to exogenous erythropoietin in HDAC5−/− mice. (A) Hematocrit (HCT) and RBC in adult WT and HDAC5−/− animals receiving weekly injections of the long-acting agent darbepoetin alfa. N = 6 animals/group. (B) Erythroid maturation in marrows of WT and HDAC5−/− mice after either no treatment (basal) or 3 injections of darbepoetin alfa (Epo). Flow cytometry for expression of CD71 and Ter119, with percentages shown within indicated quadrants. (C) Comparison of percentages of the less mature Ter119+ CD71+ progenitors and of the more mature Ter119+ CD71− progenitors. Composite of data from panel B analyzing marrows from WT or HDAC5−/− (KO) mice either untreated (basal) or darbepoetin-treated (mean ± SEM; n = 3/group).
Cell-intrinsic influence of HDAC5 on erythroid maturation
To determine whether progenitor cell-intrinsic effects contributed to the erythroid abnormalities in HDAC5−/− mice, isolated marrow progenitor cells were analyzed ex vivo. Specifically, CD71Bright Ter119− cells sorted from adult marrows were seeded in medium with abundant erythropoietin (2 U/mL), followed by flow cytometric assessment of maturation on day 3 of culture. A significant fraction of WT progenitors underwent erythroid maturation ex vivo and consisted predominantly of CD71Bright Ter119+ cells, with very few cells bearing the more mature CD71Dim Ter119+ phenotype (Figure 7A). The HDAC5−/− progenitors also underwent erythroid maturation but consistently yielded a greater proportion of the more mature CD71Dim Ter119+ cells (Figure 7A-B). These findings recapitulate the in vivo marrow response to exogenous erythropoietin, in which HDAC5−/− erythroid progenitors were more evenly distributed between CD71Bright Ter119+ and CD71Dim Ter119+ subsets (Figure 6B-C).
Enhanced ex vivo maturation of HDAC5−/− progenitors in the presence and absence of erythropoietin. (A) HDAC5−/− progenitors yield increased proportions of Ter119+ CD71Intermediate cells (R5) in erythropoietin-containing cultures. Sorted Ter119− CD71Bright progenitors from WT and HDAC5−/− adult marrows were cultured 3 days in G1ER maintenance medium with 2 U/mL erythropoietin. Flow cytometric plots show proportions of cells at indicated stages (R2-R5) of erythroid maturation, with gating on viable cells. (B) Composite of 3 experiments performed as in panel A comparing WT and HDAC5−/− (KO) progenitors (mean ± SEM). (C) Erythropoietin–independent erythroid maturation of HDAC5−/− progenitors. Flow cytometric plots from an experiment conducted as in panel A, except using medium lacking erythropoietin (gating on viable cells). (D) Composite of 3 experiments conducted as in panel C, showing percentage Ter119+ cells (mean ± SEM).
Enhanced ex vivo maturation of HDAC5−/− progenitors in the presence and absence of erythropoietin. (A) HDAC5−/− progenitors yield increased proportions of Ter119+ CD71Intermediate cells (R5) in erythropoietin-containing cultures. Sorted Ter119− CD71Bright progenitors from WT and HDAC5−/− adult marrows were cultured 3 days in G1ER maintenance medium with 2 U/mL erythropoietin. Flow cytometric plots show proportions of cells at indicated stages (R2-R5) of erythroid maturation, with gating on viable cells. (B) Composite of 3 experiments performed as in panel A comparing WT and HDAC5−/− (KO) progenitors (mean ± SEM). (C) Erythropoietin–independent erythroid maturation of HDAC5−/− progenitors. Flow cytometric plots from an experiment conducted as in panel A, except using medium lacking erythropoietin (gating on viable cells). (D) Composite of 3 experiments conducted as in panel C, showing percentage Ter119+ cells (mean ± SEM).
Previous studies of fetal liver erythroid maturation in erythropoietin receptor-deficient mice have demonstrated an erythropoietin signaling requirement for transition of progenitors from a CD71Dim to CD71Bright phenotype, followed by up-regulation of Ter119.16 To determine whether loss of HDAC5 could bypass an erythropoietin signaling requirement for maturation, progenitors isolated as in Figure 7A-B were cultured in medium lacking erythropoietin and analyzed by flow cytometry. Strikingly, HDAC5−/−, but not WT progenitors, displayed evidence of erythropoietin–independent maturation, consisting of up-regulation of Ter119 on a significant proportion of cells (Figure 7C-D). This maturation was incomplete in that the Ter119+ cells died before down-regulation of CD71. Nevertheless, these results support HDAC5 as a cell-intrinsic erythroid control element functioning downstream of erythropoietin. To determine whether these effects were exerted through regulation of GATA1 target genes, sorted CD71+ Ter119− erythroid progenitors cultured in medium without erythropoietin were analyzed by quantitative RT-PCR for expression levels of classic GATA1–activated (Ahsp) and –repressed (Kit) genes. No significant alterations were seen in HDAC5−/− progenitors, suggesting that loss of HDAC5 does not globally perturb GATA transcriptional programs (supplemental Figure 5D).
Discussion
The current findings establish an erythropoietin-PKD-HDAC5-GATA1 signaling pathway involved in erythropoietic regulation. This pathway most probably affects a subset of GATA1 molecules and a subset of GATA1 target genes (see model in supplemental Figure 6). The existence of such a pathway is supported by several prior observations. HDAC3, HDAC4, and HDAC5 directly bind GATA1 in vitro; HDAC5 represses GATA1 transactivation in reporter assays; and endogenous HDAC5-GATA1 complexes in MEL cells undergo dissociation on HMBA induction.10 Treatment of primary human progenitors with HDAC inhibitors strongly promotes erythroid lineage commitment and allows for erythroid differentiation in the absence of erythropoietin.25,26 In addition, signaling elements upstream of PKD, PLC-γ1, and PKC-ϵ are activated by erythropoietin and have been implicated in erythropoietic regulation.31,32 PLC-γ1 null mice display embryonic lethality at day 9.5-10.5 of development with complete absence of erythroid progenitors but no deficit of granulocyte or monocyte progenitors.33 The mechanistic basis for their unusual phenotype of lineage-selective erythroid aplasia has not been elucidated. PKC-ϵ, a direct activator of PKD,20 potentiates transactivation by GATA1 in reporter assays, an activity not shared by any other PKC isozymes.34 The signaling pathway identified in the current studies thus integrates several previously unexplained findings.
Our data confirm the previously described interaction of GATA1 with HDAC5 and show that this interaction may undergo disruption as a consequence of erythropoietin signaling. The mechanism responsible for dissociation from GATA1 in erythroid cells does not appear to involve global nuclear export of HDAC5, as has been described in cardiac hypertrophy.8 Alternative possibilities include the targeting of only GATA–associated HDAC5 for nuclear export, or a signal–induced shift of HDAC5 sublocalization within the nuclear compartment. Shifting of HDAC5 nuclear sublocalization has been described both in MEL differentiation and as a consequence of expressing the corepressor SMRTe.10,35 Our data suggest that HDAC4 does not contribute to GATA1 regulation in erythroid cells as a consequence of its constitutive cytoplasmic localization. This pattern of differential localization of HDAC4 and HDAC5 has been previously observed35 and remains poorly understood with regard to mechanism or functional significance.
The lineage instructional model of hematopoietic cytokine signaling has undergone challenge because of the shared repertoire of signaling pathways used by various cytokines in various lineages. For example, erythropoietin and thrombopoietin both activate JAK2-STAT5, PI3K-AKT, and Ras-MAPK pathways.36 An additional problem with the instructional model has been the paucity of mechanistic links between canonical signaling pathways and the highly specific transcriptional programs associated with lineage commitment and differentiation. Erythropoietin activation of the transcription factor STAT5 regulates growth and survival but not differentiation; mice expressing an erythropoietin receptor mutant defective in STAT5 activation display normal steady-state erythroid differentiation, and hematopoietic deletion of STAT5a/b in adult mice minimally perturbs the erythroid differentiation program.37,38 Mechanisms for canonical cytokine signaling pathways to influence the transcription factors that actively program erythroid differentiation (GATA1, SCL, and EKLF) remain undefined. Although GATA1 has been shown to undergo phosphorylation by AKT and ERK,3,4,39,40 the role of these modifications in erythropoietic regulation remains uncertain.7
One post-translational modification potentially involved in regulation of GATA factor function consists of lysine acetylation. GATA1 undergoes acetylation by p300/CBP on highly conserved lysine residues adjacent to both DNA-binding fingers.11,41 Mutation of these sites dramatically impairs transcriptional function in reporter assays, in differentiation induction in vitro, and in the programming of erythropoiesis in zebrafish.11,41,42 The mechanistic basis for GATA1 activation by acetylation remains controversial, with some studies showing direct enhancement of DNA binding and others showing no effects on in vitro DNA binding but potent regulation of in vivo chromatin binding.27,41,42 Recent experiments have shown that GATA1 acetylation on 2 sites, K312 and K315, mediates recruitment of a BET family bromodomain factor Brd3, which plays a critical role in promoting GATA1 occupancy of erythroid target genes.28,43 The other hematopoietic GATA factors, GATA2 and GATA3, also undergo functional modulation by lysine acetylation.44,45 Our results illustrate that acetylation of a hematopoietic GATA factor can undergo modulation as a result of membrane receptor signaling to HDAC modules, thus providing a mechanism for cytokine regulation of transcriptional function.
Loss of HDAC5 function in vivo significantly increased marrow BFU-E frequency and diminished CFU-GEMM frequency. These findings parallel the in vitro results of Chaurasia et al, in which treatment of human cord blood progenitors with HDAC inhibitors strikingly increased BFU-E frequency.26 The HDAC5−/− mice also displayed altered erythroid maturation patterns in response to anemic challenge and erythropoietin treatment. Studies analyzing gene expression patterns in purified marrow subpopulations have shown up-regulation of GATA1 and the erythropoietin receptor in multipotential progenitors, before erythroid lineage commitment or differentiation.46-48 However, the phase of erythroid development most highly dependent on erythropoietin signaling consists of the final round of division within the CFU-E stage, a point of transition from self-renewal divisions to differentiation divisions.16 Our data suggest that HDAC5, acting as an intermediary between the signaling and transcriptional machinery, may influence both the rate of lineage commitment and the kinetics of passage through the erythropoietin–dependent developmental transition.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mitch Weiss (Children's Hospital of Philadelphia) for the G1ER cells, Eric Olson (University of Texas Southwestern) for HDAC5−/− mice, Lisa Vohwinkle for assistance with histology, and Dennis Templeton for helpful comments.
This work was supported by the National Institutes of Health (grants DK090926 and DK079924) (A.N.G.), the Roche Foundation for Anemia Research (A.N.G.), and University of Virginia K12 Clinician Scholar Award (G.C.B.).
National Institutes of Health
Authorship
Contribution: L.L.D. and A.N.G. designed the study; L.L.D, and G.C.B. performed and planned research; L.L.D., G.C.B., and A.N.G. analyzed data; and A.N.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adam N. Goldfarb, University of Virginia School of Medicine, MR5 Building, Room 3219, 415 Lane Rd, Charlottesville, VA 22908; e-mail: ang3x@virginia.edu.