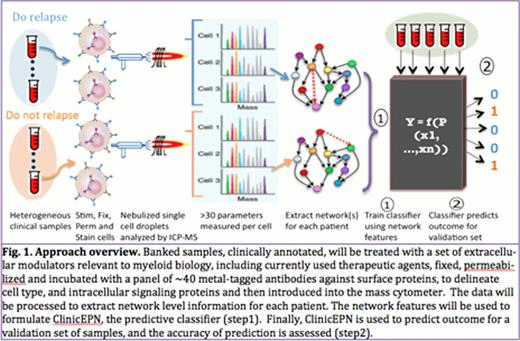
Abstract
Abstract 1411
Currently available indicators of risk stratification for acute myeloid leukemia (AML) approximate a patient's true prognosis (Rubnitz, J.E., et al., Lancet Oncol, 2010). While signaling networks are central to biological systems they do not lend themselves to easy characterization. As such, network characteristics are not incorporated into risk stratification. There exists a need for computational techniques that enable elucidation of a patient's global signaling state.
Flow cytometry is an essential tool for classifying AML surface markers by surface marker expression. Previous work has shown that intracellular signaling responses can be predictive of clinical outcome (Irish et al., Cell 2004; Kornblau et al., Clin. Cancer Res. 2010, Kornblau et al. Blood Cancer J 2011). We hypothesized that there may exist additional layers of information in both cell phenotypes and signaling responses which were not recognized in the low-parameter systems used in these studies, and which, upon characterization, may yield prognostic benefit.
31-parameter single cell mass cytometry was used to measure the simultaneous co-expression of 16 surface markers and 15 intracellular signaling epitopes in pediatric AML diagnosis bone marrow samples (n=15) and healthy adult bone marrow controls (n=3). Signaling dynamics were measured under 18 stimulation conditions including cytokines and small molecule kinase inhibitors. Single cell resolution enables statistical extraction of correlative relationships which exist among the measured variables. Network inference elucidated the regulatory signaling structure in each patient (Sachs et al. Science 2005, Itani et al. JMLR 2008), and a statistical tool called a decision tree was used to predict the patient's relapse status (Figure 1).
The analysis selects network features predictive of clinical outcome, here, relapse status. Of the network edges with a strong statistical signal, the most informative were the relationships between pS6 and pSTAT1, pAKT and cleaved Caspase3, pAMPK and pRb, pRb and pP38, pAMPK and pErk and pCREB and pCbl. Despite the small dataset size, the decision tree correctly classified >85% of the patients, an improvement over a random model which returns 50% accuracy. (The predicted value was excluded from training to avoid “memorization” by the classification model). For a small subset of the patients (3) who relapsed, paired relapse samples were also available. Strikingly, of the network features which consistently differed between the diagnostic sample and its paired relapse counterpart, two overlapped with those found to have predictive information in the above: pAMPK – pRb and pRb – pP38. These results implicate a coordination of metabolism, cell cycle and survival
Statistical classification typically relies on large numbers of profiled samples; thus the ability to predict relapse status from diagnostic samples in this small pilot study is a strong indication of the utility of this approach. Predictive accuracy relied on network features and was not achieved using univariate measurements alone. Furthermore, the features extracted by the predictive model determine which correlations help reveal the prognostic fate of a tumor at diagnosis, potentially providing mechanistic insight. These correlative relationships corroborate the roles of metabolism, survival and cell cycle in AML. The application of 31-parameter mass cytometry at the single cell level provided a level of detail that enabled characterization of signaling relationships at high resolution. This proof of principle study will inform future studies of AML signaling networks and prognostic biomarkers.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.