Abstract
Abstract 157
We report a nanoparticle-enabled therapeutic approach to B cell lymphoma using synthetic, high-density lipoprotein nanoparticles (HDL-NP). Like natural HDLs, biomimetic HDL-NPs target scavenger receptor type B-1 (SR-B1), a high-affinity HDL receptor expressed by lymphoma cells. Functionally, and unlike natural HDL, a gold nanoparticle template used to control HDL-NP synthesis enables differential manipulation of cellular cholesterol flux through SR-B1. Recent evidence in lymphoblasts and myeloblasts from patients with acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML) demonstrates enhanced uptake of cholesterol through high-density lipoprotein (HDL) carriers, which may result in increased cell proliferation. We therefore hypothesized that by targeting SR-B1, we could manipulate cholesterol flux in lymphoma cells thereby targeting cellular signaling pathways that would lead to cell death and offer an innovative approach to the treatment of lymphoma and other cancers.
To accomplish this, we developed a biomimetic spherical nanoparticle (HDL-NP) with surface chemical properties similar to natural HDL, including the ability to sequester cholesterol. Biomimetic HDL-NPs are synthesized using a 5 nm diameter gold (Au) nanoparticle (NP) as a size- and shape-restrictive template on which to assemble the surface chemical components of natural HDLs, including phospholipids and the HDL-defining apolipoprotein A1 (Apo A1). Importantly, the core AuNP template occupies the real estate in natural cholesterol-rich HDLs reserved for esterified cholesterol, which inherently limits the ability of HDL-NPs to deliver cholesterol. We incubated the HDL-NPs with various lymphoma cell lines, and similarly tested the HDL-NPs in a xenograft model.
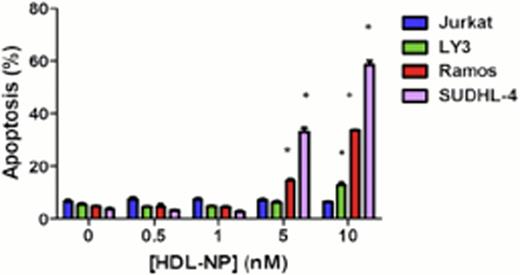
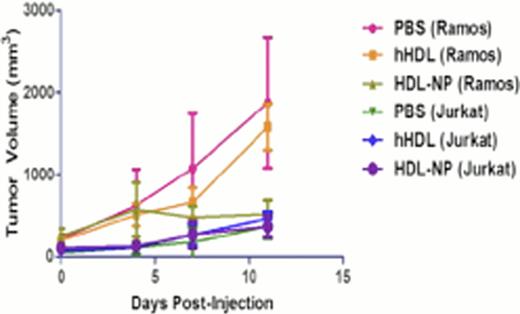
We first examined gene expression profiles of diffuse large B-cell lymphoma (DLBCL), Burkitt Lymphoma (BL) and normal B cells from patient samples in a database generated using Affymetrix U133plus 2.0 arrays in order to establish the prevalence of SR-B1 expression. We compared the expression of SR-B1 in BL cases (n=20), and DLBCL cases (n=40) that were further subdivided as activated B-cell (ABC)-like DLBCL (n=20), and germinal center (GC)-like DLBCL (n=20) to normal naive (n=3) and memory (n=3) B cells obtained from healthy donors. We found that SR-B1 was expressed at two to four-fold higher levels in the lymphomas (ABC and GC) compared with normal B cells. Next, we determined the expression of SR-B1 in lymphoma cell lines and normal peripheral lymphocytes by immunoblotting, and we found that SR-B1 is expressed in multiple B cell lymphoma cell lines, but not in Jurkat, a T-cell line, and is not expressed by normal human lymphocytes. Incubation of HDL-NP with Ramos, LY-3 and SUDHL-4 resulted in a dose-dependent decrease in cell viability and apoptosis (Figure 1) of the Ramos and SUDHL-4 cells, less so in LY-3 cells, and not in the Jurkat line. This required the nanoparticle construct and could not be duplicated by individual components of that construct, and was reversible with addition of acetylated low-density lipoprotein, indicating that the SR-B1 receptor was targeted. Xenograft experiments with SCID beige mice (C.B-Igh-1b/GbmsTac-Prkdcscid-Lystbg N7) bearing Ramos and Jurkat flank tumor xenografts confirmed the activity of the HDL-NP (Figure 2).
We report a template-directed and bio-functional therapeutic nanostructure that could shift the paradigm for treating lymphoma and other cancers. A combination of SR-B1 binding and manipulation of cholesterol flux is responsible for selective induction of apoptosis in B cell lymphoma.
Thaxton:Aurasense: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.