Abstract
Abstract 1613
Elderly patients with aggressive B-cell lymphoma are underrepresented in almost all clinical trials and treatment related toxicity mainly due to anthracycline treatment is a major concern. Individual pharmacogenetic settings may influence the prognosis due to altered efficacy and treatment related toxicity. Several single nucleotide polymorphisms (SNPs) involved in metabolization of chemotherapeutic agents have been proposed to influence the clinical course of disease in younger patients. However, no data are available in elderly patients.
We retrospectively analyzed and characterized 83 consecutively diagnosed patients ≥75 years with aggressive B-cell lymphoma (78 cases of diffuse large B-cell lymphoma and 5 cases of follicular lymphoma grade 3) from January 2004 until December 2011 treated at our tertiary cancer center by chart-based review. DNA was extracted from diagnostic tissue samples and analysis for 10 SNPs with previously reported effect on pharmacodynamics of components of the CHOP regimen were performed with commercially available primers (rs1883112 NCF4, rs3957357 GSTA1, rs4673 CYBA, rs1799977 MLH1, rs1045642 ABCB1, rs9024 CBR1, rs1695 GSTP1, rs17222723 ABCC2, rs7853758 SLC28A3, rs1056892 CBR3).
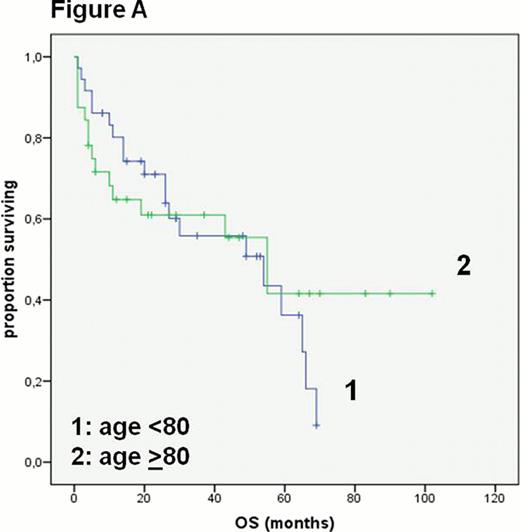
The entire cohort of 83 patients had a median age of 80 years (range 75–97 years) and 43 of 83 patients were male. Based on clinical judgment of fitness 82% of all patients received a combination of anthracycline and rituximab based therapy. The median overall survival (OS) in all patients was 43 months. Unsurprisingly, patients deemed eligible for a treatment with an anthracycline and rituximab had a better median OS than patients not eligible for this approach (54 vs 6 months p< 0.0001). Patients not treated with this combination therapy were significantly older (p<0.0001), had a worse ECOG status (p<0.03) and a higher Charlson index of Comorbidity (CI) (p<0.02). The cohort treated with an anthracycline and rituximab (n=68) had a median age of 79 years and 50% had a CI ≥1. In this group there was no significant difference in the median OS in patients <80 or >80 years of age (54 vs 55 months, Figure A) or with a CI ≤ 1 or CI ≥1 (43 vs 59 months).
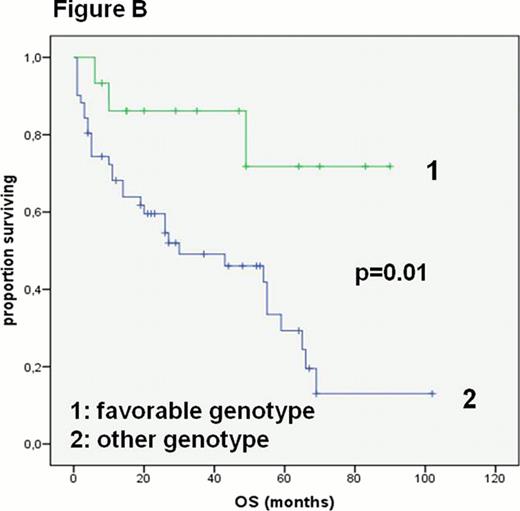
Tissue samples were available for 97% of these patients, which were used for DNA extraction and SNP analyses. Pharmacogenetic testing of 10 SNPs was performed as described before. The AA genotype of CBR 3 suggesting lower risk for anthracycline related cardiomyopathy or the GG genotype of MLH 1, which influences the mismatch repair system and the promotion of apoptosis e.g. triggered by chemotherapy, was found in 15 of 65 (23%) patients. Patients with either of these genotypes had a better median OS (30 months vs not reached p=0.01, Figure B). In multivariate analysis this benefit remained significant. Additional significant factors were an age-adjusted IPI ≤ 1 and a cumulative dosage of doxorubicin higher than 200mg/m2. The latter is likely a surrogate for overall tolerability of chemotherapy and no early progression.
Given the encouraging OS data of our unselected cohort we think that elderly patients with aggressive B-cell lymphoma should be offered curative immunochemotherapy with an anthracycline regardless of age taking into account severe comorbidities. We could further show that CBR3 and MLH1 polymorphism had an impact on the OS of these patients. The favorable CBR3 A/A genotype suggests the generation of a lower level of the cardiotoxic compound doxorubicinol after doxorubicin treatment in these patients, who are likely to be more prone to anthracycline-caused toxicity than younger patients. The impact of the MLH1 genotype indeed seems to be changed in elderly compared to younger patients, where the A/A genotype is associated with a better OS in patients treated with R-CHOP. The favorable impact of the G/G phenotype in elderly patients may be caused by a lower susceptibility to apoptosis induction in the healthy tissue. Thus, the meaning of pharmacogenetic markers may be substantially different between the young and the elderly due to the different impact of toxicity and efficacy in these populations. Based on these results a subgroup with exceptionally good OS may be defined for this age.
Melchardt:Roche: Honoraria, Speakers Bureau; GSK: Research Funding; Ratiopharm: Research Funding. Weiss:Roche: Honoraria. Greil:Roche: Honoraria, Research Funding, Speakers Bureau; GSK: Research Funding; Ratiopharm: Research Funding. Egle:Roche: Honoraria, Research Funding, Speakers Bureau; GSK: Research Funding; Ratiopharm: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.