Abstract
Abstract 1701
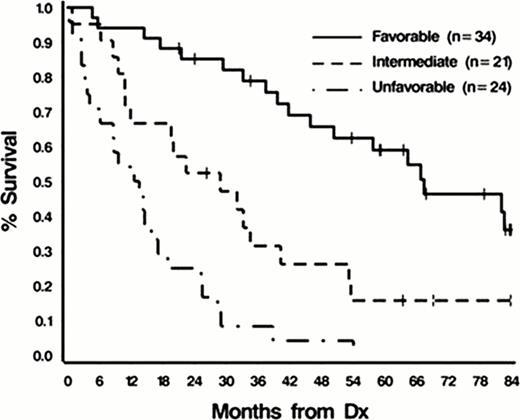
Patients with features of MDS and MDS/MPN who do not fulfill diagnostic criteria for a specific subtype of MDS and MDS/MPN are categorized by the WHO 2008 diagnostic criteria as MDS-U and MDS/MPN-U. MDS includes RCUD, RCMD, RARS, RAEB-1, RAEB-2, MDS-U and 5q- syndrome while MDS/MPN includes CMML, JMML, atypical CML and MDS/MPN-U. The natural history of patients who belong to these disease subtypes are hetergeneous. Although included in currently accepted prognostic scoring schemes like the International Prognostic Scoring System (IPSS) in MDS, Revised IPSS, and MD Anderson prognostic scoring schemes, they represent a minority of patients in the cohort. Within MDS/MPN cases, the clinical heterogeneity of diseases that belong to this group has been recognized and has led to the development of the MD Anderson prognostic Scoring System for CMML. Similarly, a prognostic scoring system for JMML has also been devised to help in risk stratification and treatment decisions. However, there are no prognostic scoring systems for unclassified cases of MDS and MDS/MPN. Clinically, we observe stark differences in treatment responses and clinical outcomes between MDS/MPN-U and other MDS/MPN-subtypes, and MDS-U with other subtypes of MDS. In total, we studied 92 patients with unclassifiable cases seen at the Cleveland Clinic, including MDS/MPN-U (n=52 [57%]) and MDS-U (n=40 [43%]). Hematologic, bone marrow (BM), cytogenetic (metaphase cytogenetic [MC]/SNP-A) and survival data were collected. Survival comparisons were made by Kaplan-Meier analyses. Cox-proportional hazard ratio was used to determine factors predictive of outcomes. A p-value of ≤0.05 was considered statistically significant. In this cohort, median age at diagnosis was 69 years (20–88), 65% (60/92) were male, and 35% (32/92) were female. Median follow-up was 21 months. Median absolute neutrophil count (ANC) was 2.69k/uL (0–87), peripheral blood (PB) blasts 0% (0–70%), hemoglobin 9.6g/dL (5–15), and LDH 260 U/L (105–2113). SNP-A karyotyping was completed for 65 patients, and new cytogenetic mutations were detected in 72% (47/65): (gains [64%], losses [57%], UPDs [25%]). In 52% (49/92) of patients, we sequenced molecular mutations that typically confer poor prognosis in myeloid neoplasms, such as ASXL1, IDH1/2, EZH2, K/NRAS, CBL and TP53. This sequencing revealed a mutational frequency of 18% (9/49) in TET2, 14% (7/49) in ASXL1, 6% (3/49) in EZH2 exons 18–19, 2% (1/49) in CBL, 2% (1/49) in NRAS, and 4% (2/49) in TP53. No mutations were found in IDH1/2 and KRAS. In univariate analysis of clinciopathologic factors, the following factors were found to be associated with overall survival: ANC (≥8.5 vs <8.5k/uL) (p<.0001), presence of PB blasts (p<.0001), presence of immature myeloid cells (p<.0001), presence of BM blasts (>3% v. ≤3%) (p<.0001), age (≥65 vs <65) (p<.0003), LDH (≥550 vs <550U/L) (p<.0004), albumin (≤3.6 vs >3.6g/dL) (p<.0008), IPSS Risk Group (Int-2/high vs int-1 vs low) (p<.01), IPSS-R Risk Group (High/very high vs low vs very low) (p<.001), WBC (≥15 vs <15k/uL) (p<.001), Hgb (≤11.5 vs >11.5g/dL) (p<.003), % BM cellularity (>85 vs ≤85%) (p<.009), and number of cytopenias (3 vs 2 vs 1 vs 0) (p<.04). In multivariate analysis, age (HR=3.47 CI 1.85–6.51, p=.001), ANC (HR=2.27 CI 1.15–4.49, p=.02), Hgb (HR=2.11 CI 1.07–4.14, p.03), peripheral blasts (HR=2.27 CI 1.19–4.36,p=.01) and LDH (HR=2.40 CI 1.11–5.16, p=.03) were independent predictors of OS in unclassifiable cases of MDS and MDS/MPN. Consequently, a prognostic scoring system was developed to include these factors. A simple scoring assigned 2 points each to an ANC of ≥8.5k/uL, the presence of peripheral blood blasts, hemoglobin ≤ 11.5g/dL, LDH ≥ 550U/L; and 3 points for age ≥65. This results in three well-separated prognostic groups: (favorable [score:0–3], median OS=67.4 months; intermediate [score:4–6],median OS=28.9 months; and poor [score: ≥ 7], median OS=13.1 months, p<.0001). 34, 21, and 24 patients were placed in these three groups, respectively. In conclusion, clinic-pathologic factors like age, LDH levels, ANC count, Hgb levels and peripheral blood blasts are helpful in predicting survival outcomes in patients with unclassifiable cases of MDS and MDS/MPN disorders. This is the first scoring system devised specifically for patients with this disease subtype.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.