Abstract
Abstract 1742
Karyotype is one of the most potent and reproducible risk factors for both overall (OS) and leukemia-free (LFS) survival in primary myelofibrosis (PMF) (Blood 2011;118:4595). It is currently not clear if the number of metaphases studied or the abnormal metaphase percentage alters this prognostic impact.
An updated Mayo Clinic database of karyotypically- and DIPSS-plus-annotated patients with PMF was used to identify a consecutive series of patients and their cytogenetic information obtained at time of referral was centrally re-reviewed. Cytogenetic results were interpreted and reported according to the International System for Human Cytogenetic Nomenclature; abnormal karyotype was defined by the presence of at least 2 metaphases with structural abnormalities or monosomy or 3 metaphases with polysomy, regardless of number of metaphases examined. For this particular study, the presence of less than 20 evaluable metaphases did not disqualify patients. “Very high risk” karyotype included monosomal karyotype, inv(3) or i(17q) abnormalities (Blood 2011;118:4595). “unfavorable” karyotype included complex or any sole or two abnormalities that included +8, −7/7q-, -5/5q-, inv(3), i(17q), 12p-, or 11q23 rearrangement (Blood 2011;118:4595). All other cytogenetic abnormalities were considered “favorable”
A total of 590 patients (median age 65 years; range 19–89 years) including 424 (72%) males. The DIPSS-plus (JCO 2011;29:392) risk distribution was 40% high, 39% intermediate-2, 12% intermediate-1 and 9% low. Cytogenetic findings included 17 (3%) very high risk, 69 (12%) unfavorable, 165 (28%) favorable and 339 (57%) normal karyotypes. The number of bone marrow metaphases studied to report these cytogenetic findings were ≥20 in 468 (79%) patients, 11 to 19 in 71 (12%) patients and ≤10 in 51 (9%) patients; the proportion of cases studied with ≥20 metaphases were 53% for very high risk, 74% for unfavorable, 83% for favorable and 80% for normal karyotype (p=0.006). Among patients with abnormal karyotype, the abnormal metaphase percentage was ≥75% in 148 (59%) patients, 50 to 74% in 36 (15%) patients, 26 to 49% in 27 (11%) patients and ≤25% in 38 (15%) patients; the proportion of patients with ≥75% was 59% for very high risk, 67% for unfavorable and 56% for favorable karyotypes (p=0.70).
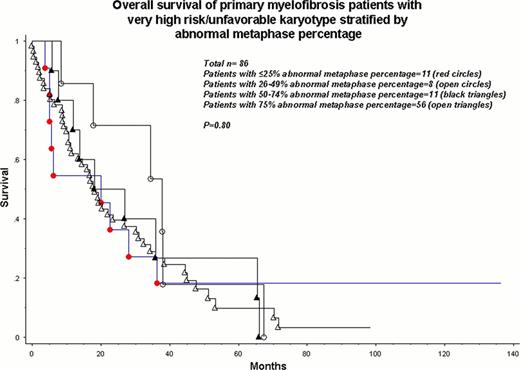
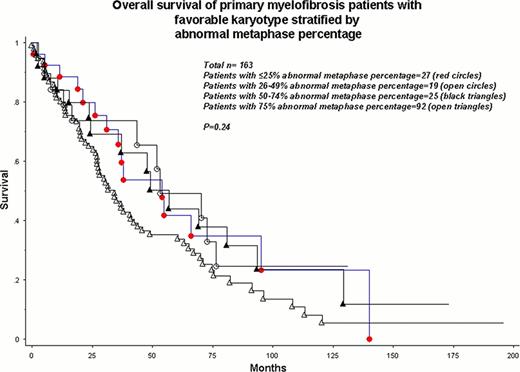
As expected, OS was significantly different among very high risk, unfavorable, favorable and normal karyotype patients with respective median survivals of 8, 23, 41 and 57 months (p<0.0001). The number of metaphases studied (p=0.62) or the abnormal metaphase percentage (p=0.12), by themselves, did not affect survival. Similarly, the survival difference among the aforementioned cytogenetic risk groups was equally apparent when patients with ≥20 metaphases studied (n=468; P<0.0001) and those with <20 metaphases studied (n=122; p<0.0001) were separately analyzed. Analysis of patients with very high risk or unfavorable karyotype (n=86) revealed no significant effect of abnormal metaphase percentage on survival (Figure; p=0.80). A similar scenario was demonstrated for patients with favorable karyotype (Figure; p=0.24).
Neither the number of metaphases examined nor the abnormal metaphase percentage appear to influence the currently recognized cytogenetic risk stratification in PMF. The current study has implications for both clinical practice and clinical research involving cytogenetic prognostication in hematological malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.