Abstract
Abstract 1821
It has been reported that cancer cells utilize glycolysis pathway (non-oxidative breakdown of glucose) even in the presence of adequate oxygen to provide cancer cells with energy, called the Warburg effect (aerobic glycolysis) that ultimately leads to produce lactate. We reported in the last ASH meeting that aerobic glycolysis is up-regulated in multiple myeloma (MM) cells in patients with high serum LDH levels and aerobic glycolysis itself could serve as a novel therapeutic target in MM patients. Here we report an importance of lactate transporter for the growth and survival of MM cells. Lactate, produced from pyruvate by lactate dehydrogenase A (LDHA), is known as an important energy source for solid tumor cells and is associated with tumor angiogenesis and chemo-resistance (Pinheiro, C., et al. J Bioenerg Biomembr. 44:127–139, 2012). On the other hand, LDHB converts lactate to pyruvate, thus negatively regulating lactate production. It is known that lactate is pumped out through monocarboxylate trasnporter, MCT4, while MCT1 mainly imports lactate to inside of cells. However, roles of MCT1 and MCT4 in MM cells remain to be elucidated. We here investigated the roles of these two molecules in the growth and survival of MM cells. CD147, a purported chaperone protein for MCT1, was also examined.
Six MM cell lines, RPMI8226, U266, KMS12BM, KMS12PE, KHM11, and KMM1 were employed. Six genes associated with glycolysis, i.e., LDHA, LDHB, MCT1-4, were examined using real time PCR analysis. Expressions of MCT1 and MCT4 were analyzed with western blotting. Expression of CD147 was investigated by flow cytometry. Lactate production into culture supernatants of MM cell lines were analyzed by using a lactate analyzer. An inhibitor of MCT1, a-cyano-4 hydroxycinnamic acid (CHC), was utilized to analyze cytotoxic effects on MM cells. AnnexinV/PI stained cells was analyzed by flow cytometry to quantify cytotoxicity. MCT1-expression was inhibited by using siRNA. Dichroloacetate (DCA), an inhibitor of PDK1, was utilized for inhibiting glycolysis.
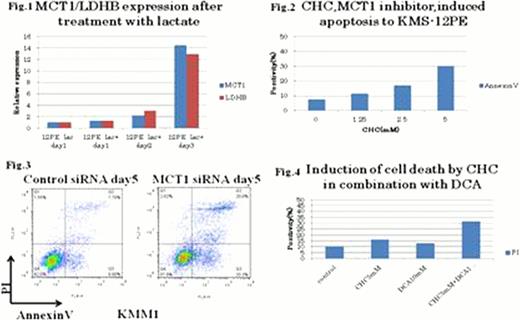
Accumulation of lactate was found in the supernatants of MM cell lines as cell density increased. Transporters of lactate, MCT1, MCT4 and CD147, were found in most MM cell lines at various levels, suggesting that transportation of lactate occurs through membrane of MM cells. To examine the role of lactate as a growth promotion factor, lactate was exogenously supplemented to KMS-12-PE cells. Interestingly, expressions of MCT1 and LDHB genes increased by the addition of lactate while those of MCT4 and LDHA only moderately changed (Fig. 1), suggesting that lactate was imported to cells through MCT1, then converted to pyrvate by LDHB. These results raised a possibility that lactate is utilized by MM cells as a growth factor. To examine the possibility, CHC, an inhibitor of MCT1, was supplemented to MM cell cultures. Interestingly, CHC induced apoptosis in MM cells in a dose dependent manner (Fig. 2). Moreover, inhibition of MCT1 gene by siRNA showed significant induction of apoptosis (Fig. 3), strongly suggesting that MCT1 plays a crucial role for survival of MM cells. Finally, we found a significant increase in the apoptosis of MM cells when CHC and DCA were simultaneously added in the culture (Fig.4), suggesting that MCT1 functions independently from glycolysis per se and that CHC and DCA act additively in starving lactate within MM cells.
Our results suggest that lactate is actively transported through monocarboxylate transporters. Given the results that exogenous lactate production increased MCT1 and LDHB expression, lactate should play a role as a regulator of lactate transportation and glycolysis as well as an important energy source. Because we found significant amount of lactate was produced from stromal cells obtained from MM patients, lactate may be supplied not only from MM cells themselves but also from micro-environment. Our finding that inhibition of MCT1 leads to cell death suggests that MCT1 could be a potential novel target molecule in MM therapy that could be stratified in combination with glycolysis inhibitor.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.