Abstract
Abstract 1836
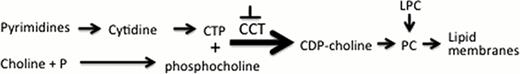
Phosphatidylcholine (PC) is the most prominent phospholipid in mammalian endoplasmic reticulum (ER) membranes. The rate-limiting step in PC synthesis through the Kennedy pathway is the conversion of phosphocholine + cytidine triphosphate (CTP) to cytidine diphosphocholine, (CDP)-choline, via the enzyme CTP:phosphocholine cytidylyltransferase (CCT) (see figure).
Multiple myeloma (MM) cells may be particularly dependent on this biosynthetic reaction because of their high consistent level of ER stress and requirement to continuously replenish ER membranes. Indeed, CCT-null mice have a defect in differentiation of B lymphocytes to plasma cells and deficiencies in Ig synthesis. To test whether this pathway remains critical in survival of malignant MM cells, we exposed MM cell lines to an inhibitor shown to inhibit CCT activity, HexPC. HexPC induced apoptosis in all MM cell lines in a concentration- and time-dependent manner. The addition of lysophosphatidylcholine (LPC), presumably converted to PC independently of the Kennedy pathway, completely rescued MM cell apoptosis. In contrast, similar concentrations of LPC in the same cell lines could not rescue apoptosis induced by bortezomib. An additional intervention to inhibit phosphatidylcholine synthesis, namely inducing pyrimidine starvation, also resulted in MM cell apoptosis and down-regulation of CDP-choline levels. Apoptosis of MM cells induced by HexPC was associated with induction of ER stress as shown by enhanced phosphorylation of IRE1 and eIF-2alpha. This ER stress was also prevented when LPC was added to HexPC although LPC could not prevent similar ER stress induced by bortezomib. These results underscore the importance of this phosphatidylcholine synthesis pathway in MM cells and provide new targets for future therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.