Abstract
Abstract 2016
The role of AHSCT in AL amyloidosis is controversial. There is evidence that AHSCT offers long term disease control with high organ response rates, however, the only randomized phase III trial comparing transplant with conventional melphalan and dexamethasone therapy deemed transplant inferior. Better understanding of risk stratification for SCT candidacy has resulted in TRM rates are as low as 5.6% in some series. The goal of the current study was to evaluate the TRM rate for all consecutive patients who have undergone AHSCT for AL amyloidosis at institution. Special attention in evaluation of any detectable differences in TRM over the time period of this review was a major goal of this project.
Retrospective review was performed of all patients with primary AL amyloidosis who have undergone AHSCT at our institution. The database captured patients from December 2001 and our study includes patients through February 2012. Patients who were diagnosed with secondary amyloidosis by the stem cell transplant service and/or referring oncologist were excluded. TRM was defined as death from any cause within 100 days post stem cell infusion. We then evaluated data from the entire cohort and divided it into two different time periods: 2001–2006 and 2007–2012.
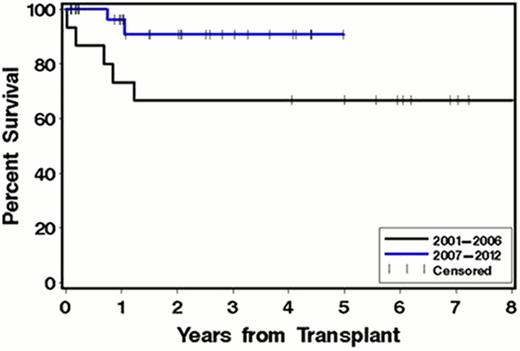
Thirty-nine patients had an initial AHSCT during the study period. Nine patients were enrolled in the SWOG 0115 trial which comprised tandem autoSCT using 100 mg/m2 of melphalan conditioning 3–6 months apart. Of those, 6/9 (66%) had both transplants. For purposes of this abstract we report on the data resulting from first transplantation only for patients in the SWOG 0115 trial. The median age at transplant for the entire cohort was 57.6 years old (39–74). Males represent 64% of the patients. Most of the patients had symptomatic involvement of one organ (48.7%) with 23% having more than 2 organs involved. There were 14 patients transplanted in the 2001–2006 period and 25 patients in the 2007–2012 period. Overall TRM for 2001–2012 was 5.1%. During the 2001–2006 period TRM was 14% (2/14 patients). No TRM occurred for patients transplanted from 2007–2012. Overall survival (OS) improved when separately comparing the two study periods. Overall survival was 64% at 3 years in the 2001–2006 cohort and 92% at 3 years in the 2007–2012 cohort.
Patients in the 2007–2012 cohort include all patients enrolled to SWOG 0115. Sixty percent of patients reported for that period received <200mg/m2 melphalan for transplant conditioning. Eight of nine patients enrolled in SWOG 0115 received 100 mg/m2, one received 140mg/m2. Six patients treated on our institution's standard amylodosis SCT protocol received 140mg/m2 conditioning. The remaining ten patients received 200mg/m2 melphalan. Patients in the 2007–2012 period were more likely to receive induction treatment prior to transplant with novel regimens (72% vs 21% p=0.003). In addition, these patients were more frequently diagnosed with amyloid cardiac involvement. For purposes of this abstract amyloid cardiac involvement is defined according to whether or not the SCT team concluded on the basis of history, physical examination and available ancillary data (including echocardiography) that the patient had amyloidotic cardiac disease (40% vs 14% p=0.15). All patients had echocardiography pre-AHSCT. The mean Inter Ventricular Septum (mIVS) thickness was 14.5 mm in the 2007–2012 period vs. 11.7 mm in the 2001–2006 one p=0.004.
Univariate analysis of risk factors affecting improved OS identified year of transplant (>2006; p=0.03), number of treatments pre-transplant (>1; p=0.04), and time from diagnosis to transplant (>4mo; p=0.05) as factors associated with improved survival. Number of organs involved (>1; p=0.06) showed a trend towards worse survival.
In conclusion, our single institution data demonstrates a reduction in TRM over the past 5 years. Reasons for improvement in TRM are under investigation and may include better risk stratification, better supportive care (including frequent hospitalization for cardiac monitoring in high-risk patients) and improvement in pre-AHSCT performance status for those patients receiving induction therapy with novel agents.
Comparison of overall survival of patients with AL amyloidosis after autologous stem cell transplant based on year of transplant.
Comparison of overall survival of patients with AL amyloidosis after autologous stem cell transplant based on year of transplant.
Holmberg:Millenium: Research Funding; Otsuka: Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.