Abstract
Abstract 2479
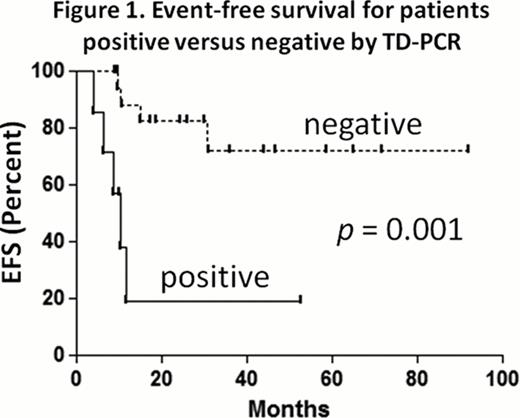
FLT3/ITD mutations are present in approximately one quarter of adult AML cases. Patients with these mutations carry a particularly poor prognosis. For this reason, a number of small molecule FLT3 inhibitors are being evaluated in clinical trials. In AML patients with FLT3/ITD mutations, it has become an increasingly common practice to pursue allogeneic bone marrow transplantation quickly during first remission. Among this patient population, as with other AML patients, relapse following transplant occurs frequently. As a result, there are numerous ongoing efforts to investigate a potential role for maintenance therapy with FLT3 inhibitors in the post-transplant setting. Historically, post-transplant remission in AML patients has been assessed using bone marrow morphology as well as hematologic recovery. Better methods are needed to assess minimal residual disease in AML patients. The standard method of detecting the FLT3/ITD mutation (Murphy KM, J Mol Diagn 2003) with PCR has a limit of detection of approximately 1 in 100 cells. In many FLT3/ITD AML patients who ultimately relapse with FLT3-mutated disease after transplant, the FLT3 mutation is still not detectable by standard PCR assay at time points before and after transplant, because their burden of disease is lower than the detection threshold of the assay. We report here a new PCR technique for detecting the presence of a FLT3/ITD mutation with a high level of sensitivity and specificity. The technique is referred to as tandem duplication PCR (TD-PCR). Instead of using inward-facing primers that flank the juxtamembrane domain of FLT3, TD-PCR uses outward-facing, overlapping primers within the expected region of mutation. The TD-PCR primers will not amplify any product when annealed to a wild type FLT3 template. However, if a tandem duplication is present, the primers will anneal at two distinct sites and will amplify a product, provided the duplication is long enough (roughly 30 to 40 base pairs). A duplication under this size does not allow enough room to anneal both primers. To investigate the potential clinical utility of TD-PCR, we performed a chart review at our institution and found that between December 2004 and May 2012 there were 62 patients diagnosed with FLT3/ITD AML who subsequently underwent allogeneic bone marrow transplant while in morphologic remission. Of those 62 patients, 50 were alive and in remission 60 days after the transplant. DNA samples prepared from bone marrow collected on or around Day 60 following the transplant were available for 36 of these 50 patients. Based on the size of the duplications, the TD-PCR assay was informative for 26 of these 36 (72%). Of the 26 patients whose TD-PCR results were informative, seven (27%) had positive TD-PCRs on their day 60 bone marrow specimens. The standard clinical PCR was performed on six of these seven patients and was negative in all six cases. Of the seven patients with positive post-transplant TD-PCRs, five (71%) have relapsed to date, while two still remain in remission (one of whom is on sorafenib maintenance). Nineteen of the 26 (73%) had negative TD-PCRs on their day 60 bone marrow specimens. Of these 19 patients, only two (11%) have relapsed thus far, one of whom relapsed with FLT3 wild type AML and one of whom relapsed with the FLT3/ITD mutation 29 months after the transplant. TD-PCR is thus highly predictive (p = 0.001; log-rank) for relapse risk for FLT3/ITD AML following allogeneic transplant (Figure 1). It is possible that this technique will identify patients who might benefit from post-transplant therapy such as FLT3 inhibition or donor lymphocyte infusion. We are currently examining the use of TD-PCR prospectively in an ongoing clinical trial using post-transplant sorafenib in FLT3/ITD AML patients. Larger studies are necessary to validate this technique as a prognostic tool and as a means of guiding therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.