Abstract
Systemic mastocytosis (SM) is a neoplasm of mast cells (MC) and MC progenitor cells. The clinical picture in SM ranges from an indolent course to highly aggressive cases with short survival time. In a majority of all patients with SM, the KIT mutation D816V is detectable. In addition, activating RAS mutations have recently been identified in patients with advanced SM. So far, no curative therapy for advanced SM is available. To identify molecular targets in neoplastic MC, we have generated novel human MC lines by lentiviral immortalization of cord blood-derived MC progenitors with RAS G12V, SV40 large T antigen, and hTERT. These cell lines, designated MCPV1 through MCPV4, display a morphology and ultrastructure resembling immature MC progenitors. MCPV cells also express a number of MC differentiation antigens, such as KIT and tryptase, and produce an aggressive MC disease when injected into NOD-SCID IL-2RG−/− (NSG) mice. As assessed by flow cytometry, MCPV cells were found to express a number of different surface antigens, including CD13, CD30, CD33, CD44, CD52, CD54, CD63, and CD95. In consecutive analyses, we studied the cell surface target CD52, also known as CAMPATH-1 antigen. As assessed by FACS, CD52 was found to be expressed on primary neoplastic MC in patients with aggressive SM (ASM, n=3), whereas in all patients with indolent SM (ISM, n=6), neoplastic MC stained negative or only slightly positive for CD52. Normal MC stained negative for CD52 in these experiments. Expression of CD52 in neoplastic MC was also demonstrable by immunohistochemical staining of bone marrow biopsy sections. We then asked whether the putative stem cells in ISM and ASM would express CD52. Indeed, in all patients examined, including cases with ISM and ASM, the immature CD34+/CD38- cells were found to express CD52. Next, we studied the regulation of expression of CD52 in neoplastic MC. Since activating RAS mutants have been described to be expressed in neoplastic MC in patients with advanced SM, we asked whether oncogenic RAS would promote expression of CD52 in MC. Indeed, lentiviral-mediated expression of activated RAS mutants was found to upregulate mRNA expression and to promote surface expression of CD52 in the human MC line HMC-1 as well as in various other myeloid cell lines. To validate CD52 as a potential target in neoplastic MC, we applied the CD52-targeting drug alemtuzumab. In these experiments, alemtuzumab exerted only minimal direct effects on viability of MCPV cells after IgG-mediated crosslinking. However, incubation of MCPV cells with alemtuzumab induced rapid and dose-dependent cell death via complement-dependent cytotoxicity (Figure 1). Furthermore, alemtuzumab was found to induce complement-dependent cytotoxicity in CD52+ primary neoplastic MC obtained from patients with advanced SM (n=2). In conclusion, we have established a new powerful model for studying the biology of advanced SM. Moreover, our study has identified CD52 as a novel promising therapeutic target in neoplastic MC and MC progenitor cells. Whether targeting of neoplastic (stem) cells in advanced SM using alemtuzumab will improve therapy by eradicating malignant cells remains to be determined in clinical trials.
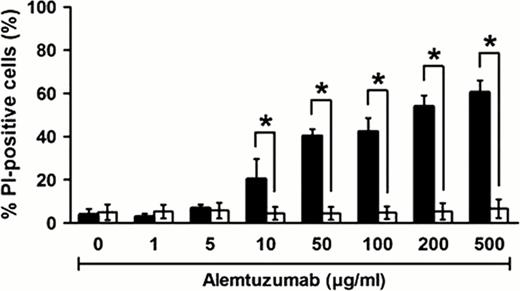
Alemtuzumab induces complement-dependent cytotoxicity in MCPV cells. MCPV cells were incubated with alemtuzmab and human serum as a source of complement (black bars). Heat-inactivation of the serum (white bars) abolished the cytotoxic effect.
Alemtuzumab induces complement-dependent cytotoxicity in MCPV cells. MCPV cells were incubated with alemtuzmab and human serum as a source of complement (black bars). Heat-inactivation of the serum (white bars) abolished the cytotoxic effect.
Valent:Phadia: Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.