Abstract
Abstract 3917
VEGF is an important pro-angiogenic factor involved in survival and dissemination of chronic lymphocytic leukemia cells. At high concentrations such as those present in bone marrow niches, VEGF reduces MMP9 production and favours bone marrow retention. But on the other hand, it is well known that autologous VEGF and CD49d are required for B-CLL cell migration. In other cancer cell lines, this effect seems to be mediated by CXCR4/SDF-1 axis. In this work we studied the role of exogenous VEGF on B-CLL migration as well its relationship with the G-coupled proteins CXCR4, CXCR7 and CD49d.
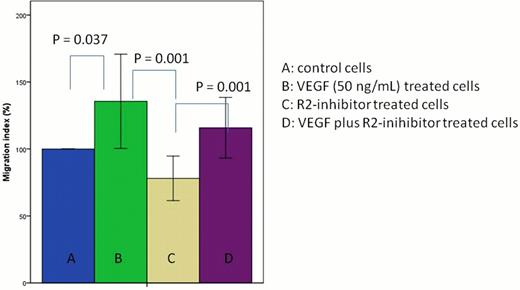
We obtained peripheral blood mononuclear cells from 33 patients diagnosed of CLL according to established clinical and laboratory protocols at our institution after informed consent. We also used the CLL cell line Mec-1. B-lymphocytes were purified by Ficoll-Hypaque density gradient centrifugation anti-CD19 conjugated Dynabeads and stored in liquid nitrogen. Then we analysed by flow cytometry the expression of several chemokines and adhesion molecules, CXCR4, CXCR7 and CD49d before and after exogenous VEGF exposure (50 ng/mL), VEGF-R2 inhibitor (70 nM) or both. In order to evaluate the role of VEGF in the motility of these cells, we performed an in vitro 24-hours transmigration assay towards a media containing (or not) SDF-1. B-CLL (5 × 105 cells) cells were incubated on the upper chamber of transwell filters coated with human umbilical vein endothelial cells in the presence or absence (control) of vascular endothelial growth factor (VEGF) ([50 ng/ml], 24 hours), VEGFR2/KDR inhibitor [70nM] or both simultaneously. We quantified the migration ratio between those stimulated with VEGF compared with non-stimulated controls, inhibitor-treated cells versus control and both (R2-inh plus VEGF) versus inhibitor-treated cells. Migration ratio was given as mean ± SD. Statistical analysis was performed by non-parametric Wilcoxon test using SPSS statistical software (version 19.0).
Basal CXCR4, CXCR7 and CD49d expression levels of B-CLL cells were highly variable among the 33 patients analyzed. Mean fluorescent intensity (MFI) of CXCR4 expression was significantly higher on cells treated with VEGF versus untreated cells (average increase, 9.64 ± 95, p=0.028). However, we did not detect a significant difference in the percentage of cells expressing this receptor. On the other hand, VEGF treatment did not influence either the mean fluorescent intensity or the number of CXCR7 and CD49d expressing cells. Exposure to a VEGFR2 inhibitor reduced the percentage of cells expressing CXCR4 and CXCR7, suggesting a potential regulatory role of this receptor in the expression of these chemokines. Concerning B-CLL migration, we observed a significant increase in cell migration of cells treated with exogenous VEGF versus the control ones in both the Mec-1 cell line and the primary cells (27.66 ± 69.97 p= 0.03). Furthermore, the treatment with VEGFR2 inhibitor reduced significantly the migration index (−23.18 ± 33.5, p= 0.001) and the motility was restored by the addition of VEGF to the R2-inhibitor treated cells (36.04 ± 39.33, p=0.001).
This preliminary data suggest that VEGF seems to be involved in B-CLL migration through the regulation of CXCR4 expression levels. Detailed molecular mechanisms implicated in this process should be further studied. New therapeutic strategies focussed in blocking both the SDF1-CXCR4 axis and/or VEGF pathway could have a potential therapeutic implication by decreasing B-CLL cell migration into lymph nodes and bone marrow microenvironment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.