Abstract
Abstract 3932
The best initial treatment for elderly patients (pts) with CLL is evolving and is a field of active investigation. We conducted a phase II study evaluating the activity of lenalidomide as initial therapy for elderly pts with CLL. This treatment was associated with an overall response rate of 65% and an overall survival of 88% at 2 years (Badoux, Blood 2011). Because response duration is a relevant endpoint, we performed an analysis looking at long-lasting responses to lenalidomide in pts on this trial.
Pts with a response lasting 36 months or longer were defined as “long-term responders” (LTRs) and are the focus of our report. Clinical characteristics, prognostic factors, serum immunoglobulin levels, circulating T cell numbers (up to 36 months) and plasma cytokine levels of LTRs were compared with the rest of the study population using non-parametric and Chi-square tests. Differences were considered to be significant if p was < 0.05.
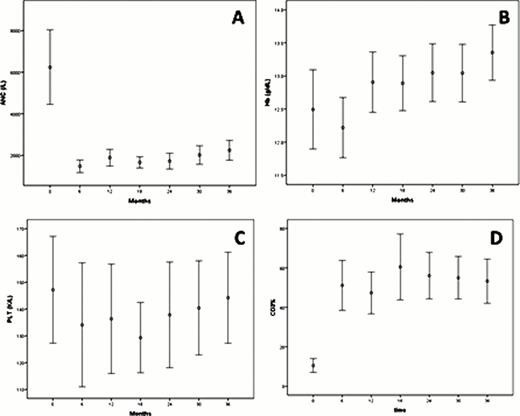
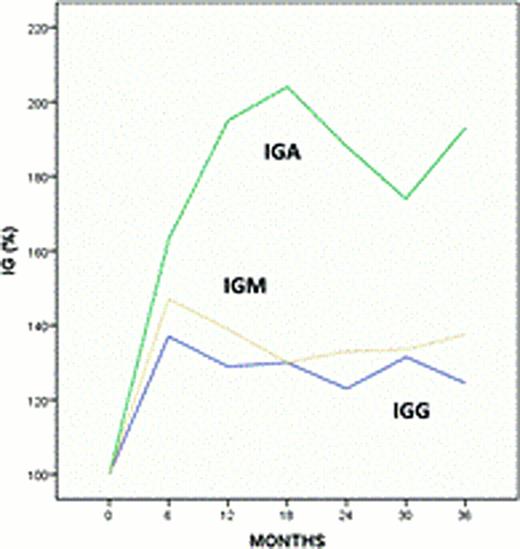
Thirty-one of the 60 pts (52%) are LTRs. Best responses among LTRs consisted of 29 complete remissions (CR), including 4 pts with MRD-negative CR, and 2 partial remissions. Median time to failure (TTF) has not been reached for LTRs, after a median follow up of 47 (37–53) months. The median daily dose of lenalidomide at last follow-up in these pts was 5 mg (2.5–10). Twenty-two LTRs are still on therapy and nine have discontinued lenalidomide. Reasons for treatment discontinuation were: toxicity in 6 pts (deep venous thrombosis after 41 months in 1 pt, moderate neuropathy after 30 and 39 months in 2 pts, persistent fatigue after 23 months in 1 pt, moderate weight loss after 5 months in 1 pt, immune thrombocytopenia after 11 months in1 pt), infectious complications occurred in 1 pt (sepsis, after 12 months), second malignancy (invasive squamous cell carcinoma of the skin after 26 months) in 1 pt and change of institution in 1 pt. LTRs experienced neutropenia during the first 12 months of therapy that later resolved in 83% of pts (Fig 1A); additionally a recovery in hemoglobin and platelets compared to baseline values was also seen in 100 and 77% of LTRs, respectively (Fig 1B-C). We also observed an increase in the percentage of circulating T cells (CD3+) (Fig 1D) and an increase in plasma levels of immunoglobulins A, G and M values in these pts (Fig 2). Lenalidomide-related late toxicities observed in LTRs consisted of G 1–2 diarrhea in 2 pts and G1 peripheral neuropathy in 3 pts. One patient developed several skin lesions shown to be in situ squamous and basal cell carcinomas. When we compared pre-treatment clinical characteristics of LTRs and the other pts on study, we observed that LTRs had lower baseline beta-2-microglobulin (median values: 4 vs 4.8 mg/L; p=0.02) and were less likely to have a deletion 11q (5 vs 9; p=0.02). Furthermore, baseline plasma levels of IL6, IL8, IFNγ and MIP1α were significantly lower in the LTRs (p=0.002, 0.025, 0.044 and 0.001, respectively).
In conclusion, lenalidomide as initial therapy of elderly pts with CLL induced responses lasting more than 3 years in 53% of pts. Compared to the pts with shorter response duration, LTRs were more likely to have lower baseline levels of beta2-microglobulin, IL6, IL8, IFNγ and MIP1α and intermediate or favorable cytogenetic abnormalities.
Keating:Celgene Corporation: Consultancy. Wierda:Celgene Corporation: Consultancy. O'Brien:Celgene Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Ferrajoli:Celgene Corporation: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.