Abstract
Abstract 4187
Acute GVHD (aGVHD) is the major early complication following allogeneic hematopoietic cell transplantation (HCT). Of patients developing grade (Gr.) 2–4 aGVHD, only 50–60% achieve a complete response to systemic steroids. Well-designed clinical trials with endpoints for treatment success are required to test new therapies to improve aGVHD outcomes. We studied the previously described day (d) 28 response to steroids (Martin et al BBMT 2009, MacMillan et al Blood 2010) and the newly proposed ASBMT 6 month (m) freedom from steroid failure (FFSF) (Martin et al BBMT 2012) as predictors of survival for patients with aGVHD treated with systemic steroids.
Adult patients with hematological malignancies undergoing T cell replete HCT from February 2007 to March 2009 and enrolled in a prospective regulatory T cell (Treg) biomarker clinical trial, who developed aGVHD requiring treatment with systemic steroids within the first 100 days were included (N=44). aGVHD response to steroids at d28 after treatment initiation was assessed. FFSF at 6m was defined per ASBMT guidelines [death, malignancy relapse/progression, or systemic immunosuppression (IST) change within 6m of starting steroids and prior to classic chronic GVHD (cGVHD) diagnosis]. cGVHD was treated as a competing risk. Treg subsets were measured at engraftment and d30. Overall survival (OS) and treatment related mortality (TRM) were calculated from the start of steroids.
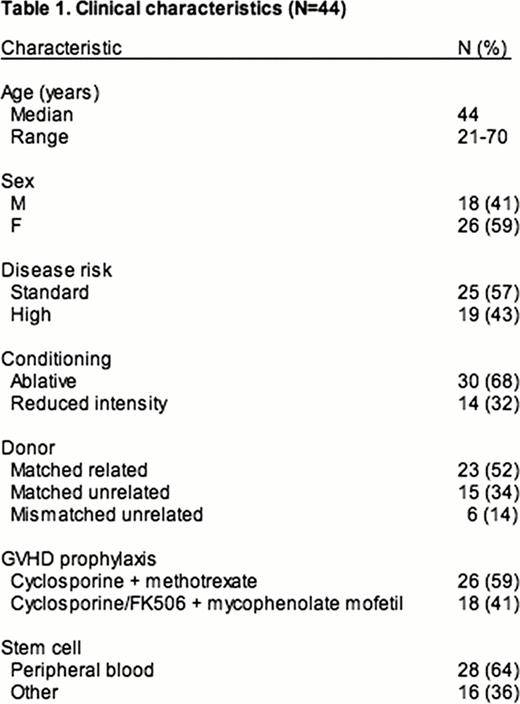
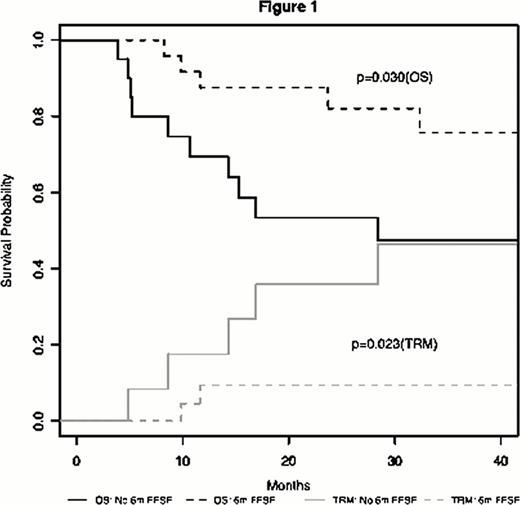
aGVHD requiring systemic steroids occurred in 44 patients [Gr. 1 (N=2), Gr. 2 (N=30), Gr. 3–4 (N=12)] (Table 1). Skin only, gut only, and multi-organ aGVHD affected 7 (16%), 19 (43%), and 18 (41%) patients, respectively. D28 response to steroids was CR [N=14 (32%)], VGPR [N= 7 (16%)], PR [N= 17 (39%)], and NR [N= 6 (13%)]. Within 6m of steroid initiation, 17 (39%) patients had new systemic IST added, 11 (25%) were diagnosed with cGVHD, 8 (18%) relapsed, and 4 (9%) died (causes of death were relapse=3, TRM=1). Thus, 38 (86%) patients responded (CR+VGPR+PR) to treatment by d28 after steroid initiation, but only 24 (55%) patients met the 6m FFSF endpoint. The median follow-up for surviving patients (N=29) was 3 years (y) (range, 0.5–4 y). Death from relapse and TRM occurred in 8 (18%) and 7 (16%) individuals, respectively. Transplant outcomes were improved in patients responding to steroids (CR+VGPR+PR) when compared to those with NR at d28 [2y OS of 75% vs. 22% (P=0.021) and 2y TRM of 11% vs. 73% (P=0.007)]. FFSF at 6m was associated with superior 2y OS (88% vs. 53%; P=0.030) and decreased TRM (9% vs. 36%; P=0.023) when compared to individuals failing steroids by 6m (Figure 1). We modified the original ASBMT criteria for 6m steroid failure to include 5 additional patients who had steroid dosage increased within 6m of starting initial treatment or who had steroid doses ≥0.25 mg/kg at d180 but this did not improve the predictive power of the endpoint [OS (P=0.125) or TRM (P=0.175)]. After adjusting for age and disease risk, both d28 response (HR, 0.24; 95%CI, 0.07 to 0.82; P=0.022) and 6m FFSF (HR, 0.29; 95%CI, 0.09 to 0.96; P=0.042) were associated with improved OS. The median frequency of Foxp3+ Tregs at engraftment was higher in patients who responded to steroids at day 28 (4.66 vs. 3.38; P=0.044) and in those achieving 6m FFSF (6.04% vs. 3.76%; P=0.021), suggesting a possible biological mechanism for these endpoints.
D28 response and 6m FFSF are predictive of transplant outcomes. Given the differing kinetics of response to therapeutics used for aGVHD, it is likely that both d28 response and 6m FFSF are required as clinical trial endpoints. The strength of the 6m FFSF endpoint is that it incorporates fixed elements in determining response, thus decreasing subjectivity. If validated, these endpoints could be used in future therapeutic trials and subsequently facilitate FDA approval of new aGVHD treatments
Overall Survival and Treatment Related Mortality Stratified by 6 Month Freedom from Steroid Failure (6m FFSF).
Overall Survival and Treatment Related Mortality Stratified by 6 Month Freedom from Steroid Failure (6m FFSF).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.