Abstract
Abstract 4278
Pediatric acute myeloid leukemia (AML) survival rates have improved primarily because of treatment protocol advancement via cooperative-group clinical trials. Contrary to the consistency in chemotherapy protocols, there is often a lack of consensus for supportive care therapies leading to significant variation in practice. Invasive fungal infections are a major cause of treatment-related morbidity and mortality in pediatric AML. However, the lack of pediatric-specific guidelines for antifungal therapy has the potential to result in either under- or over-utilization of antifungal agents across institutions. We aimed to explore the variability in antifungal use across hospitals caring for children that were treated for de novo AML with a homogeneous induction regimen.
The Pediatric Health Information System (PHIS) administrative database was used to establish a cohort of children treated for de novo AML between 1999 and 2010 at one of 43 freestanding children's hospitals across the United States. Only institutions with 15 or more AML patients during the study period were considered in the final analysis. Patients were included if they were assigned an ICD-9 discharge diagnosis code for myeloid or unspecified leukemia and if their daily pharmaceutical billing data supported the receipt of an ADE induction regimen (cytarabine, daunorubicin, and etoposide with or without gemtuzumab ozogamicin). The induction period was defined as the start of the first to the initiation of the third course of chemotherapy.
Each institution's inpatient induction period antifungal use, including azoles, echinocandins and amphotericin products, was reported as days of antifungal use per 1000 induction hospital days. A negative binomial regression analysis was performed to adjust rates of antifungal use by demographic variables (age, gender and race) and frequency of severe illness days. Severe illness days were defined by the need for intensive care resources such as intubation, mechanical ventilation or vasopressors. The all-cause induction case fatality rate for each institution was also determined.
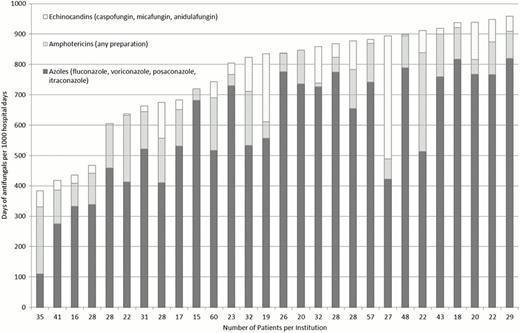
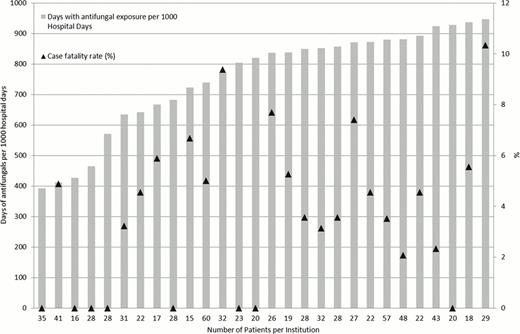
We identified 931 ADE-treated AML patients from 38 children's hospitals with an overall induction case fatality rate of 3.8%. Variation in antifungal use was defined for 28 institutions caring for 815 patients. (Figure 1) Antifungal use exposure days varied widely across institutions ranging from 384 per 1000 hospital days to 958 per 1000 hospital days (median: 836 per 1000 hospital days). Azoles were most commonly used (591 per 1000 hospital days) followed by amphotericin products (120 per 1000 hospital days) and echinocandins (60 per 1000 hospital days). After adjusting for demographic characteristics and the frequency of severe hospital days, variation in antifungal use persisted across institutions, ranging from 393 per 1000 hospital days to 946 per 1000 hospital days. (Figure 2) Induction case fatality rates also varied by institution but did not correlate with antifungal use (Spearman's rho=0.28, p=0.15) (Figure 2).
Wide variability in inpatient antifungal use for children with AML exists across pediatric institutions in the United States, but this variability does not appear to correlate with induction case fatality rates. Additional studies are necessary to define the ideal approach to optimizing antifungal therapy for children with AML.
Antifungal use during AML induction across U.S. children's hospitals, by antifungal class
Antifungal use during AML induction across U.S. children's hospitals, by antifungal class
Antifungal use during AML induction across U.S. children's hospitals, adjusted for institutional demographic variables and frequency of severe illness days
Antifungal use during AML induction across U.S. children's hospitals, adjusted for institutional demographic variables and frequency of severe illness days
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.