Abstract
Abstract 4376
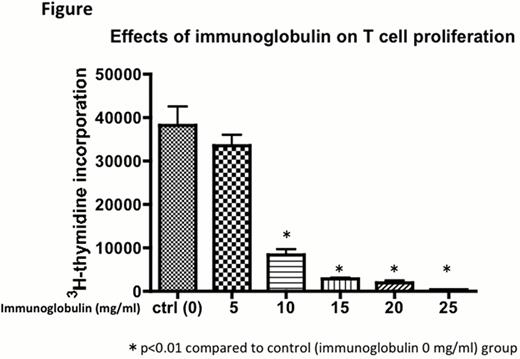
High-dose intravenous immunoglobulin (IV-IG) is used to treat many autoimmune and inflammatory disorders, yet precise mechanisms have not been understood. In organ transplant, some studies demonstrated that IV-IG therapy, even moderate-dose, given to highly sensitized patients led to reduction in allo-sensitization, fewer acute rejection episodes, and better long-term results for cardiac and renal allograft recipients. Although historically IV-IG has been widely used for mainly prophylaxis of CMV infection in hematopoietic stem cell transplantation, only a few reports mentioned that IV-IG contributed to reduce GVHD severity. The aim of this study is to explore whether IV-IG has immunomoduraroty role against cells involved in GVHD developments or not, if so, appropriate concentration of immunoglobulin and administration schedule for clinical settings should be discussed. First, mixed lymphocyte reaction (MLR) was performed using irradiated dendritic cells (DCs) from C57BL6 as stimulators and splenocytes from Balb/c as responders with immunoglobulin. MLR was strongly inhibited when the concentration was above 25 mg/mL. Next, to identify when immunoglobulin give immunomoduratory function to DCs or T-cells, or both, immunoglobulin treated DCs or splenocytes for 24h before MLR were prepared and washed to remove extra immunoglobulin, then MLR was performed without addition of immunoglobulin. MLR was not suppressed when not added to immunoglobulin during reactions, suggesting that sustained immunoglobulin interaction to DCs, T-cells, or both could be required for sustained immunomoduratory effects. Next, to clarify whether IV-IG inhibit T-cell proliferation via DCs or T-cells directly, we stimulated splenocytes (Balb/c) with phytohemagglutinin (PHA) in the presence of various concentrations of immunoglobulin. Immunoglobulin inhibited PHA-stimulated T-cells, with similarity to the result of MLR using DCs as stimulators as shown in the figure below. It suggested that IV-IG mainly act on T-cells directly, not to DCs. Similar results were obtained using human samples. Finally, to elucidate whether immunoglobulin induce arrest of T-cell proliferation with increased apoptotic cells or not, we measured the early apoptotic cells using annexin V and 7-AAD staining combined with CFSE proliferation assay. Only a few early apoptotic cells were increased when immunoglobulin was added to PHA-stimulated T-cells, demonstrating that IV-IG immunomoduratory effects were mainly brought about T-cell arrest. In conclusion, high concentration of immunoglobulin, in which only high dose IV-IG could be achieved in vivo, contributes to profound and sustained inhibition of T-cell activation. To use IV-IG to prevent GVHD in clinical settings, much higher dose of IV-IG than ever might be needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.