Abstract
Abstract 4534
Linezolid is an oxazolidinone antimicrobial often used to treat resistant gram-positive bacteria. Linezolid has been associated with mild, reversible, time-dependent myelosuppression, including thrombocytopenia, anemia, leukopenia, and pancytopenia. The hematologic effects most commonly reported are thrombocytopenia and anemia. In general, these effects have occurred with treatment durations of ≥ 14 days. Patients with underlying hematologic abnormalities may be more at risk for the development of linezolid-induced myelosuppression, but this is controversial. We hypothesized that use of linezolid before engraftment may delay hematopoietic recovery following stem cell transplant (SCT), and performed a matched controlled analysis to investigate this hypothesis.
With approval from our institutional review board, we retrospectively evaluated 24 patients who received linezolid and compared them to 60 controls who did not receive linezolid from 1/1/1997 to 1/1/2010. Our SCT database was utilized to find matched controls and matching was based on the following: diagnosis; transplant type (autologous, matched sibling, matched unrelated, cord blood); cell source (peripheral blood, bone marrow, cord blood); transplant conditioning regimen; and age within 10 years. Patients then underwent further screening for study enrollment. Patients in the linezolid group were included if linezolid was administered at any time from the stem cell infusion (day 0) through engraftment of white cells but for a duration of at least 72 consecutive hours. Patients 1 year of age or older were included. The data were analyzed for the effects of linezolid on time to neutrophil (first of 3 consecutive days in which ANC > 500) and platelet engraftment (first of 7 consecutive days in which platelet count was> 20,000 without transfusion), and the cumulative incidence of engraftment of both neutrophil and platelets within the first 100 days post-transplant. We also studied the subgroup of controls that received vancomycin to treat gram-positive infections.
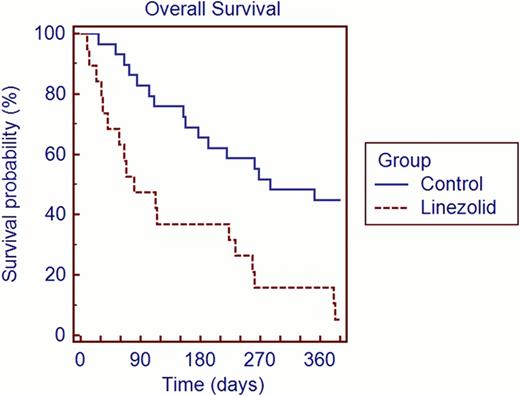
The linezolid and control groups were similar with respect to age (median 44 vs. 41 years), gender (50% vs. 58% male and 50% vs. 42% female) disease type (29% vs. 25% had AML or MDS), cell source (67% vs. 63% apheresis product), transplant type (67% vs. 63% allogeneic), ablative vs. non-ablative conditioning (79% vs. 83% myeloablative), and cell dose (median CD34 dose 4.52 × 106/kg vs. 4.34 × 106/kg). Table 1 shows engraftment data. The median time to engraftment (ANC plus platelets) for linezolid group vs. control group was 50 days (11 patients censored) vs. 15.5 days (10 patients censored). With regard to engraftment failures, 16% of patients in the linezolid group vs. 7% in the control group failed to reach ANC >500. Forty-six percent of patients in the linezolid group vs. 13% in the control group failed to achieve platelet engraftment >20,000. Figure 1 shows overall survival. Day 100 survival rates: 58% for the linezolid group vs. 92% for controls.
The cumulative incidence of engraftment of both neutrophils and platelets (using death without engraftment as the competing risk) was significantly lower in the linezolid group (54%) compared to the control group (83%) (p=0.005)
Linezolid does not significantly affect time to neutrophil engraftment, but it does appear to significantly prolong time to platelet engraftment when compared to patients who did not receive linezolid. Linezolid should be used cautiously early after stem cell transplantation.
Engraftment
| . | Linezolid group (n=24) . | All controls (n=60) . | Controls receiving vancomycin (n= 43) . |
|---|---|---|---|
| Linezolid duration, median (range) | |||
| Doses of linezolid | 19.5 (8 to 84) | – | – |
| Days on linezolid | 10 (4 to 29) | – | – |
| ANC and PLT goals, median (range) | |||
| Days to ANC ≥ 500 | 12 (8 to 43) | 12 (7 to 62) | 12 (7 to 62) |
| Days to ANC ≥ 1000 | 12 (10 to 45) | 13.5 (9 to 67) | 13 (9 to 67) |
| Days to PLT ≥ 20,000 | 18 (7 to 54) | 12 (2 to 219) | 13 (2 to 219) |
| Days to PLT ≥ 50,000 | 30.5 (13 to 128) | 19.5 (11 to 277) | 19 (11 to 277) |
| Engraftment failures, n(%) | |||
| Failed to reach ANC ≥ 500 | 4 (16%) | 4 (7%) | 3 (7%) |
| Failed to reach PLT ≥20,000 | 11 (46%) | 8 (13%) | 6 (14%) |
| . | Linezolid group (n=24) . | All controls (n=60) . | Controls receiving vancomycin (n= 43) . |
|---|---|---|---|
| Linezolid duration, median (range) | |||
| Doses of linezolid | 19.5 (8 to 84) | – | – |
| Days on linezolid | 10 (4 to 29) | – | – |
| ANC and PLT goals, median (range) | |||
| Days to ANC ≥ 500 | 12 (8 to 43) | 12 (7 to 62) | 12 (7 to 62) |
| Days to ANC ≥ 1000 | 12 (10 to 45) | 13.5 (9 to 67) | 13 (9 to 67) |
| Days to PLT ≥ 20,000 | 18 (7 to 54) | 12 (2 to 219) | 13 (2 to 219) |
| Days to PLT ≥ 50,000 | 30.5 (13 to 128) | 19.5 (11 to 277) | 19 (11 to 277) |
| Engraftment failures, n(%) | |||
| Failed to reach ANC ≥ 500 | 4 (16%) | 4 (7%) | 3 (7%) |
| Failed to reach PLT ≥20,000 | 11 (46%) | 8 (13%) | 6 (14%) |
ANC = absolute neutrophil count; PLT = platelets
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.