Abstract
Abstract 716
CLL patients characterized by 17p-, TP53 mutation or refractoriness to fludarabine (F)-based therapy show a very poor prognosis (“ultra high-risk CLL”). Although alemtuzumab (A) showed efficacy in these cohorts, the rate and duration of remissions remain unsatisfactory. Aim of the CLL2O study was to achieve higher overall response rates (ORR) by adding high-dose dexamethasone (D) to A during induction and investigating the consolidation effect of prolonged A or allogeneic stem-cell transplantation (allo-SCT), respectively.
Induction treatment consisted of subcutaneous A, 30 mg weekly × 3 for 28 days, combined with oral D, 40 mg on days 1–4 and 15–18, and prophylactic pegfilgrastim 6 mg on days 1 and 15. Treatment duration was up to max. 12 weeks. Consolidation was at discretion of pt and physician with either allo-SCT or maintenance A with 30 mg every 14 days for up to 2 years (y).
Between Jan. 2008 and Dec. 2011, 131 eligible pts were recruited at 26 centers. Pts were generally subdivided into three cohorts: 61 pts were refractory (i.e. no response or relapse within 6 months) to regimens containing F or similar (i.e. pentostatin, cladribine, bendamustine). Non-refractory pts all exhibited 17p- and had either untreated (n=42) or relapsed CLL (n=28). The median age was 66.5/64/66 y in 17p- 1st line, 17p- relapse, and F-refractory pts, respectively. The three cohorts had 45/57/77% Binet C, 40/32/31% B symptoms, 38/40/56% ECOG 1 or 2, median TK levels of 36/49/26 U/L, median β2MG levels of 3.8/5.3/4.7 mg/L, and unmutated IGHV in 90/93/87%. In the F-refractory group, 49% exhibited 17p- and 20% had 11q-. Pretreated patients had received a median of 3 (F-refractory) or 2 prior lines (17p- relapse) and 93/72% had received rituximab. 5 pts had previously undergone autologous and 1 pt allo-SCT.
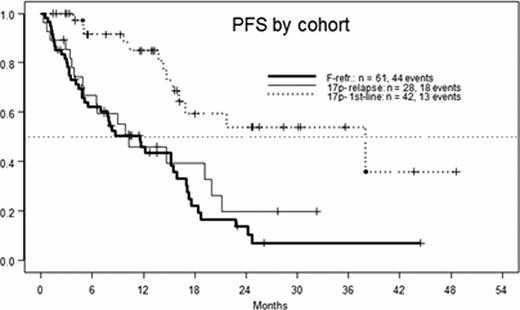
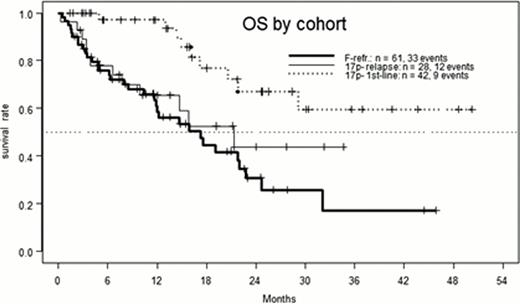
This analysis includes data for induction therapy of all eligible pts (n=42/28/61). Of these 74/57/54% received the full induction of 36 A applications, with a median duration of 11.6 weeks (range 0.6 – 33.4). ORR was generally high with 98/79/70%. Current CR rates are 19/4/3%, in part due to missing bone marrow examination (5/0/1 pts). After a median follow-up of 21 months (mo), median progression-free survival (PFS) was 38/10.3/11.6 mo. Median overall survival (OS) was not reached (>36 mo) in the 1st line group, and 21.3/17.3 mo in 17p- relapse/F-refractory pts. Consolidation treatment was maintenance A (median duration 34 weeks, range 2 – 112) in 37%, and allo-SCT in 27%, with a median age of 69 and 57.5 y in these subgroups. The main reasons for going off-study without consolidation were death due to infection (12%, n=16, of these 13 in the F-refractory cohort and 10 without response), PD (10%), and toxicity (10%). Among the 47 pts not receiving consolidation, there were 32 (68%) deaths, 23 of them in the F-refractory cohort. When comparing A maintenance and allo-SCT for consolidation, there were 23 (49%) and 12 (35%) PD events, respectively, with a trend favoring SCT (median 24.7 vs. 17 mo, p = 0.13).
During induction, grade 3/4 consisted of anemia 30%, neutropenia 46%, thrombopenia 42%, and non-CMV infection 24% of 17p- 1st-line, 29% of 17p- relapsed, and 38% of F-refractory pts. CMV reactivation was observed in 55/33/43%, without severe sequelae. During A maintenance, grade 3/4 toxicity consisted of neutropenia in 43% pts and thrombopenia in 6% pts with 10 SAEs (ITP, diarrhea, infection (2), septic shock, erythema, tachycardia and cardiac decompensation, syncope, pulmonary embolism and thrombosis), reported for 7 pts (4/1/2 pts). So far, 7 pts with consolidation by allo-SCT have died, of these 5 due to infection, 1 due to PD (Richter transformation), and 1 due to complications of acute GVHD.
The combination of A and D shows relatively high CR and ORR, with promising preliminary findings for PFS and OS, as compared to ORR/CR of 68%/5%, and median PFS of 11.3 mo in the 17p- subgroup of the FCR arm of the CLL8 study. On the other hand, in F-refractory CLL, the higher ORR does not seem to translate into a major improvement of long-term results, when compared to single-agent A. Allo-SCT may offer PFS and OS superior to maintenance A, but confirmation of this trend requires more mature follow-up and multivariate analysis.
Stilgenbauer:Genzyme: Consultancy, Honoraria, Research Funding. Ibach:WiSP CRO: Employment.
Author notes
Asterisk with author names denotes non-ASH members.