To the editor:
The NUP214-ABL1 fusion gene is found amplified as multiple (5-50) episomal copies in 6% of T-cell acute lymphoblastic leukemia (T-ALL).1,2 Alterations of the TLX1, TLX3, CDKN2A/B, and NOTCH1 genes are commonly associated with NUP214-ABL1 T-ALL. Recently, the NUP214-ABL1 fusion gene has been reported in 2 of 15 cases of B-cell acute lymphoblastic leukemia (B-ALL)3 identified by RNA-sequencing with no evidence of episomal amplification, suggesting an intrachromosomal rearrangement. In 1 of 2 cases, phosphoflow analysis demonstrated increased CRK-like protein phosphorylation, suggesting active ABL1 signaling, that was sensitive to tyrosine kinase inhibitors (TKIs). We now report the first case of B-ALL associated with extrachromosomal, episomal amplification of NUP214-ABL1. All methods can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
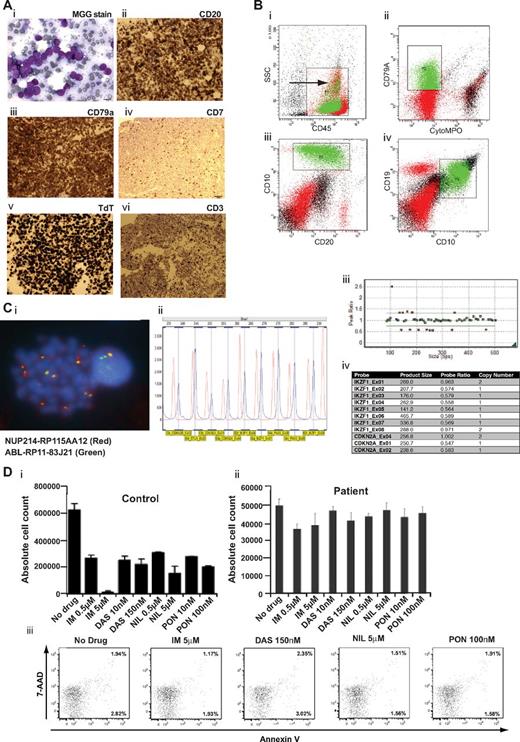
A 22-year-old male presented with a hemoglobin 108 g/L, white cell count 12.72 × 109/L, and platelet count 65 × 109/L. Bone marrow aspirate and trephine revealed 99% blast cells expressing CD79a, CD19, CD10, CD20, surface IgM, HLA-DR, cytTdT, and CD7 (Figure 1A-B). The latter 2 markers are more suggestive of bi-lineage blasts, but there was no cytCD3 CD2, CD4, CD5, CD7, CD8, cytMPO, CD33, CD13, CD15, and CD117 expression. The karyotype was: 47,XY,+X.
Characterization of episomal NUP214-ABL1 B-ALL and in vitro sensitivity of patient blasts to TKI. (Ai) May-Grunwald-Giemsa stain of blasts from a trephine roll. (ii-vi) Immunocytochemical stains of trephine sections stained with antibodies indicated above each panel. (Bi-v) Flow cytometric analysis of marrow blasts. (i) Cell populations in the blast cell gate (CD45+ and of the indicated side scatter (SSC) were studied further in subsequent panels. (ii-iv) Expression of the indicated cell surface and cytoplasmic (cyto) antigens was studied on blast cell populations. (Ci) FISH analysis of a blast cell with probes specific for ABL1 (green) and NUP214 (red). Two green and red signals indicate normal chromosomal ABL1 and NUP214. Yellow signals indicate location of fusion gene. (ii-iv) MPLA analysis shows deletion of IKZF1 exons 2-7 and CDKN2A exons 1-2. (D) Absolute cell counts of viable control BV173 (i) and patient primary blasts (ii) after 72 hours in culture with either no drug or the indicated concentration of imatinib (IM), dasatinib (DAS), nilotinib (NIL), or ponatanib (PON). (iii) FACS plots of aliquots of patient's cells after 72 hours of culture showing annexin V and 7-amino-actinomycin D (7-AAD) expression.
Characterization of episomal NUP214-ABL1 B-ALL and in vitro sensitivity of patient blasts to TKI. (Ai) May-Grunwald-Giemsa stain of blasts from a trephine roll. (ii-vi) Immunocytochemical stains of trephine sections stained with antibodies indicated above each panel. (Bi-v) Flow cytometric analysis of marrow blasts. (i) Cell populations in the blast cell gate (CD45+ and of the indicated side scatter (SSC) were studied further in subsequent panels. (ii-iv) Expression of the indicated cell surface and cytoplasmic (cyto) antigens was studied on blast cell populations. (Ci) FISH analysis of a blast cell with probes specific for ABL1 (green) and NUP214 (red). Two green and red signals indicate normal chromosomal ABL1 and NUP214. Yellow signals indicate location of fusion gene. (ii-iv) MPLA analysis shows deletion of IKZF1 exons 2-7 and CDKN2A exons 1-2. (D) Absolute cell counts of viable control BV173 (i) and patient primary blasts (ii) after 72 hours in culture with either no drug or the indicated concentration of imatinib (IM), dasatinib (DAS), nilotinib (NIL), or ponatanib (PON). (iii) FACS plots of aliquots of patient's cells after 72 hours of culture showing annexin V and 7-amino-actinomycin D (7-AAD) expression.
Interphase fluorescence in situ hybridization (FISH) using a BCR-ABL1 probe demonstrated 50-80 extrachromosomal copies of ABL1; FISH probes targeting 3′ regions of ABL1 and NUP214 confirmed episomal NUP214-ABL1 amplification in ∼ 99% of cells (Figure 1C). Conversely, a probe targeting the 5′ region of ABL1 showed a normal signal pattern. This signal configuration is the same as previously shown in T-ALL.2 Multiplex ligation–dependent probe amplification (MLPA) confirmed amplification of both ABL1 and NUP214 (data not shown). Although FISH, MPLA, and SNP6.0 analysis showed no rearrangement of TLX1 and TLX3, aberrant TLX1/3 expression cannot be excluded. MLPA showed focal deletions of exons 2-7 of IKZF1 and exons 1-2 within the CDKN2A/B locus. SNP6.0 analysis confirmed NUP214 and 3′ ABL1 amplification (supplemental Figure 1). SNP data also confirmed IKZF1 and CDKN2A/B loss and showed other copy number aberrations including some previously implicated in B-ALL; FHIT,4 TBL1XR1,5 and the histone cluster at 6p226 (supplemental Table 1).
The patient was treated with induction therapy (vincristine, daunorubicin, pegylated asparaginase, prednisolone, and CNS prophylaxis http://www.ctsu.ox.ac.uk/research/mega-trials/leukemia-trials/ukall-2003/). A day 29 marrow showed complete morphologic remission, but PCR-based IgVH MRD rearrangement studies revealed 1 in 104 cells with clonal IgVH rearrangement. The patient successfully underwent cyclophosphamide/total body irradiation myeloabalative sibling donor allogenic stem cell transplantation and is in complete remission at 4 months.
NUP214-ABL1–positive patient primary cells were cultured with low and high concentrations of imatinib (0.5 and 5μM), dasatinib (10 and 150nM), nilotinib (0.5 and 5μM), and ponatinib (10 and 100nM; Figure 1D; concentrations based on IC50 values and maximally achievable plasma concentrations in chronic myeloid leukemia patients). After a 72-hour culture, compared with no drug control, there was no significant reduction in viable cell numbers and no increase in apoptosis in any experimental arm. In contrast, BCR-ABL1 lymphoblastic cell line BV-173 demonstrated anticipated TKI sensitivities. Moreover, in T-ALL the mutant fusion kinase appeared more sensitive in vitro than BCR-ABL1 to TKIs.7 It is noteworthy, there are only case reports documenting mixed clinical responses to TKIs in NUP214-ABL T-ALL.8,9
It is unclear to what extent subtypes of NUP214-ABL1 seen in T-ALL, and now described in B-ALL, differ biologically. In part, these differences in drug sensitivity may arise kinase amplification in our case and differential kinase activation between NUP214-ABL1 and BCR-ABL1.7 Differences may arise not only from variable NUP214-ABL1 copy number, but also the nature of cooperating mutations. In our case, these likely include IKZF1 and the CDKN2A/B locus, important B-ALL aberrations. This variable genetic landscape may, in part, account for differential in vitro sensitivity to TKIs.
Authorship
The online version of this article contains a data supplement.
Acknowledgments: P.V. acknowledges funding from the Medical Research Council (MRC) Molecular Hematology Unit, MRC Disease Team Award, the Leukemia Lymphoma Research Specialist Program Grant 08030, Cancer Research UK Program Grant C7893/A12796, and the National Institute for Health Research (NIHR) Oxford Biomedical Research Center based at Oxford University Hospitals Trust, Oxford, United Kingdom. M.C. acknowledges funding from the Scottish Funding Council (Fellowship SCD/04) and Leukemia and Lymphoma Research (grant 11017). C.S., A.V.M. and C.J.H. acknowledge Leukemia Lymphoma Research Specialist Program Grant 11004.
Contribution: T.E., A.P., A.M., T.L., and P.V. collected clinical and laboratory data; C.J.S. performed genetic analysis; R.K. and M.C. performed kinase sensitivity assays and analyzed the data; T.L.H., A.V.M., and C.J.H. analyzed laboratory data; J.S. and A.K.M. performed some of the cytogenetic investigations; T.E. and P.V. wrote the manuscript; and all authors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paresh Vyas, MRC Molecular Haematology Unit, WIMM, Oxford OX3 9DU, Oxford, United Kingdom; e-mail: paresh.vyas@imm.ox.ac.uk.
References
National Institutes of Health
Author notes
T.E., C.J.S., and R.K. contributed equally to this work.