α-granules are by far the most abundant platelet granules. Yet little is known about how they are formed. In this issue of Blood, Urban et al now characterize platelets from patients with an inheritable α-granule defect, demonstrating a role for VPS16B in α-granule biogenesis and taking us one step closer to understanding how these elusive organelles are formed.1
Platelet granules were first observed in the late 19th century, when newly developed staining procedures were applied to the recently described platelet. With the application of electron microscopy to platelet biology in the 1960s, the diversity of platelet granules was first appreciated. Three classes of granules were described based on morphology: dense granules, lysosomes, and α-granules. Dense granules are endowed with membrane transporters and accumulate high concentrations of calcium, bioactive amines, adenine nucleotides, and polyphosphate. Lysosomes contain enzymes involved in protein, carbohydrates, and lipid degradation. But the dominant platelet granule in number and total volume is the α-granule. They are approximately 10-fold more numerous than dense granules and comprise roughly 10% of the platelet volume. α-granules contain hundreds of different types of proteins, which may be sorted and stored into different subpopulation of α-granules. Diverse functions have been proposed for α-granules, including roles in hemostasis and thrombosis, inflammation, antimicrobial host defense, angiogenesis, and progression of malignancy.2
Despite the prominence of α-granules in platelet biology, little is known about their biogenesis. Many proteins involved in dense granule formation have been identified by studying dense granule defects in patients and mice with various degrees of occulocutaneous albinism and bleeding diatheses. In contrast, α-granule deficiency is extremely rare. Gray platelet syndrome (GPS) is the most well-recognized disorder of α-granules, but only 1 to 2 dozen families with GPS have been identified. Nonetheless, Kahr's group and others have recently demonstrated that mutations in NBEAL2, which encodes neurobeachin-like protein 2, cause GPS.3-5 Even before this work, however, Kahr and colleagues had characterized another syndrome associated with α-granule deficiency termed arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome.6 ARC syndrome is a rare autosomal recessive condition characterized by death in the first year of life. In addition to their other maladies, these patients have platelets that lack α-granules. The fact that ARC syndrome is caused by a defect in VPS33B implicated this protein in α-granule formation.6
Urban et al have now gone on to identify another protein involved in α-granule formation: VSP16B. The group first used a yeast 2-hybrid system to identify binding partners for VPS33B. They pulled out an uncharacterized gene product, C14orf133, which they subsequently identified as human VPS16B. Additional studies confirmed an association of platelet VPS33B with VPS16B. While conducting these experiments, a paper was published demonstrating that mutations in C14orf133 were associated with ARC syndrome.7 This observation provided proof-of-principle that not only did VPS16B bind VPS33B, but this interaction was functionally important for α-granule formation.
Urban et al were able to identify a patient with ARC syndrome secondary to a homozygous mutation in VPS16B. Platelets from the patient were gray on blood films and lacked α-granules when evaluated by electron microscopy. Both α-granule specific cargo and membrane proteins were markedly decreased or absent in the VSP16B-deficient platelets. In addition, VPS33B levels were decreased, suggesting a protective function for VPS16B. In contrast to α-granules, dense granule numbers as measured by whole-mount electron microscopy were increased from 1.4/platelet in neonatal controls to 10.3/platelet in the patient with ARC syndrome. Evaluation of VPS16B localization in a megakaryocytic cell line demonstrated co-localization with markers of the trans-Golgi network, late endosomal compartment, and α-granules themselves (see figure). VPS16B did not localize with the cis-Golgi, the early endosomal compartment, or recycling endosomes.
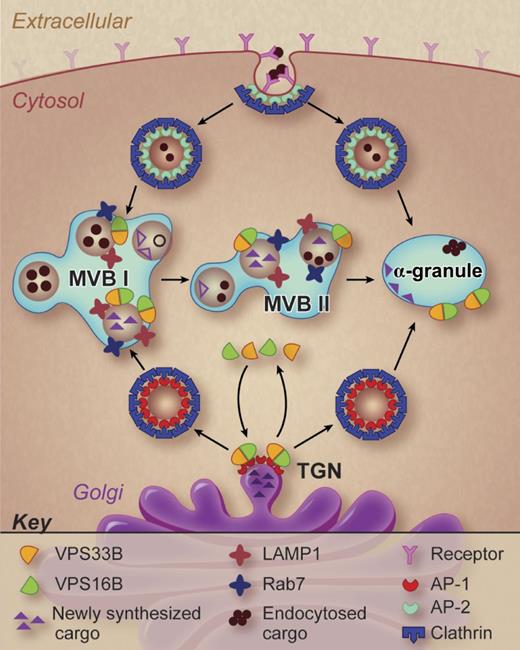
A working model of α-granule formation in megakaryocytes. Potential pathways of α-granule biogenesis are shown and possible sites of VPS33B and VPS16B action are indicated. Professional illustration by Debra T. Dartez.
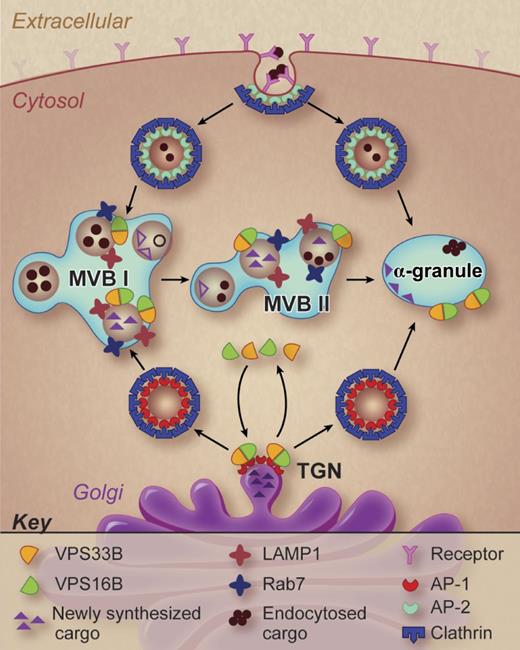
A working model of α-granule formation in megakaryocytes. Potential pathways of α-granule biogenesis are shown and possible sites of VPS33B and VPS16B action are indicated. Professional illustration by Debra T. Dartez.
These studies demonstrate that a VPS33B-VPS16B complex participates in α-granule formation. But what is the function of this complex? The precise answer will require further studies. However, some general inferences can be made from the work of Urban and colleagues. α-granules are derived from both an endocytotic pathway and a biosynthetic pathway (see figure). The VPS33B-VPS16B complex is involved in the biosynthetic pathway. Cargo proteins in the biosynthetic pathway cluster at the trans-Golgi network where adaptor proteins and coat proteins such as clathrin facilitate the pinching off and delivery of transport vesicles to the late endosomal compartment (also referred to as a multivesicular body). Multivesicular bodies I and II have been described in megakaryocytes (see figure), distinguished by the maturity of the granules they contain.8 The VPS33B-VPS16B complex may serve a sorting function, tracking with transport vesicles destined to contribute to mature α-granules. VPS33B (a Sec-1/Munc18 family protein) and VPS16B (a golgin domain containing protein) appear to interact with SNAP receptors (SNAREs) and Rab isoforms, perhaps as part of a larger homotypic vacuole fusion and vacuole protein sorting (HOPS) complex, to sort specific cargos to discrete destinations. The specific interactions that facilitate this putative sorting function need to be established.
Nonetheless, from what was a black box just a few years ago, a story of how α-granules are formed begins to emerge. VPS33B-VPS16B and neurobeachin-like protein 2 appear to shuttle developing granules toward α-granule development, while adaptor protein-3 and biogenesis of lysosome-related organelle complexes (BLOCs) shuttle developing granules toward dense granule development. The observation that α-granules are totally absent from ARC platelets, whereas empty α-granules with some membrane protein can be observed in GPS platelets, suggests that the VPS33B-VPS16B complex acts earlier in the pathway than neurobeachin-like protein 2. Similarly, it appears that in the absence of VPS33B and VPS16B (but not neurobeachin-like protein 2) immature vesicles are diverted to a dense granule pathway, substantially increasing the number of dense granules. Whether VPS33B and VPS16B acts in a larger complex is certainly worth evaluating. If so, the identity of its component proteins will provide some hints as to which SNARE and Rab isoforms function in α-granule formation. In addition to affinity-based approaches, the development of megakaryocytic vesicle trafficking models to study platelet granule formation9 will enable identification of additional components involved the generation of α-granules. Perhaps with the first of the main actors identified, a more complete story of α-granules formation is now attainable.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health