Abstract
The reactivation of human cytomegalovirus (HCMV) poses a serious health threat to immune compromised individuals. As a treatment strategy, dendritic cell (DC) vaccination trials are ongoing. Recent work suggests that BDCA-3+ (CD141+) subset DCs may be particularly effective in DC vaccination trials. BDCA-3+ DCs had however been mostly characterized for their ability to cross-present antigen from necrotic cells. We here describe our study of human BDCA-3+ DCs in elicitation of HCMV-specific CD8+ T-cell clones. We show that Fcgamma-receptor (FcγR) antigen targeting facilitates antigen cross-presentation in several DC subsets, including BDCA-3+ DCs. FcγR antigen targeting stimulates antigen uptake by BDCA-1+ rather than BDCA-3+ DCs. Conversely, BDCA-3+ DCs and not BDCA-1+ DCs show improved cross-presentation by FcγR targeting, as measured by induced release of IFNγ and TNF by antigen-specific CD8+ T cells. FcγR-facilitated cross-presentation requires antigen processing in both an acidic endosomal compartment and by the proteasome, and did not induce substantial DC maturation. FcγRII is the most abundantly expressed FcγR on both BDCA-1+ and BDCA-3+ DCs. Furthermore we show that BDCA-3+ DCs express relatively more stimulatory FcγRIIa than inhibitory FcγRIIb in comparison with BDCA-1+ DCs. These studies support the exploration of FcγR antigen targeting to BDCA-3+ DCs for human vaccination purposes.
Introduction
Viral reactivation, for example of human cytomegalovirus (HCMV), poses a major threat in patients receiving hematopoietic and solid organ transplantation, decreasing 3-year survival rates from an estimated 80%-90% to 50%.1-3 Strategies that increase early antiviral adaptive immune responses after transplantation are therefore under exploration, which could ultimately help to establish full clearance of, and long-term immunologic memory against viruses. The induction of adaptive immune protection against, for example HCMV, requires the display of antigen by professional antigen presenting cells (APCs) to lymphocytes. Considering CD8+ T-cell responses, antigen display via peptide/class I MHC complexes requires the processing of whole antigen into peptide-size fragments. Especially dendritic cells (DCs) are equipped with cell-biologic mechanisms supporting antigen processing and presentation, rendering them potent APCs.4 In mice, DC subsets can be divided alongside their various specialties, including antigen uptake, processing and presentation to T cells, and migratory or resident properties in the body.5-7 Mouse DCs expressing CD8α are specialized at antigen cross-presentation, the process by which exogenous antigen is presented as peptide/class I MHC complexes.8 This process is pivotal in antiviral and antitumor immune responses. In the human setting, tumor cell–based trials are underway, geared toward cross-presentation and activation of tumoricidal CD8+ T cells.9
Human BDCA-3+ (CD141+) DCs are considered a human counterpart of mouse CD8α+ DCs, and are found at low frequencies in peripheral blood, lymph nodes, bone marrow, and spleen.10-12 Human BDCA-3+ DCs internalize dead cell material and cross-present exogenous soluble or cell-associated proteins to CD8+ T cells.11-13 Recombinant soluble HCMV pp65 antigen was cross-presented with increased efficiency by BDCA-3+ DCs to antigen-specific CD8+ T cells.12 For vaccination strategies, full grasp on the uptake and processing mechanisms and preferentially a further increase in CD8+ T-cell stimulation potency is desired, which was the aim of our study.
In whole organisms, blood-borne soluble antigen is readily opsonized by serum opsonins, most prominently components of the complement pathway and Ab, which facilitates antigen exposure to DCs as IgG-antigen immune complexes. Immune complexes are particularly formed for antigens that are community-born and to which antibody titers are routinely present, including HCMV.14 Antigen targeting to specific receptors, including Fcgamma-receptors (FcγR) directs antigen presentation toward the class I MHC or the class II MHC presentation pathway.3,15-17 Here, we addressed the possible role of FcγR-mediated antigen targeting as a mechanism within human BDCA-3+ DCs. FcγR-mediated antigen targeting in BDCA-3+ DCs, as we show, is particularly effective at potentiating antigen cross-presentation ability in these DCs, a process that is fully blocked when FcγR function is absent. FcγR-mediated cross-presentation of the HCMV antigen involves proteolysis in both the endosomal pathway and the proteasome. Thus, FcγR antigen targeting in BDCA-3+ DCs could be exploited in HCMV vaccination strategies to counteract viral reactivations related to organ and stem cell transplantation.
Methods
Monocyte-derived DC culture
Peripheral blood mononuclear cells (PBMCs) from healthy HLA-A2 positive donors were separated from peripheral blood by ficoll isopaque density gradient centrifugation (GE Healthcare Bio-Sciences AB) and were used either directly, or frozen until further experimentation. For DC induction, PBMCs were incubated at 37°C and 5% CO2 for 1 hour to plastic for the monocytes to adhere, in X-vivo 15 medium (Lonza) containing 2% human serum (Invitrogen). Cells were washed 3 times with PBS (RT) and subsequently cultured for 5 days at 37°C and 5% CO2 in X-vivo 15 medium containing 450 U/mL GM-CSF (Immunotools) and 300 U/mL IL-4 (Immunotools). Cytokines were refreshed after 3 days. DCs were collected for experiments on day 5 by incubation in PBS (4°C) for 1 hour.
Primary DCs
Primary blood DCs were isolated from HLA-A2–positive PBMC buffy-coats. PBMCs were separated by ficoll isopaque density gradient centrifugation (GE Healthcare Bio-Sciences AB) and were subsequently depleted for CD3, CD14, CD19, and CD20 with magnetic-based cell sorting (Miltenyi MACS). Next, cells were labeled with anti–human antibodies (Abs; CD1c, CD3, CD11c, CD14, CD19, CD20, CD56, CD141, HLA-DR) and sorted by the FACS aria II (BD Bioscience) into a 96-well plate (Thermo; supplemental Figure 1A gating strategy, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Tonsillar DCs were extracted from tonsils originating from anonymous patients who underwent a tonsillectomy. Mononuclear cells were separated by ficoll isopaque density gradient centrifugation (GE Healthcare Bio-Sciences AB) and were subsequently sorted for CD141 positivity by magnetic cell sorting (Miltenyi MACS).
T-cell cloning
An HLA-A*0201–restricted, HCMV pp65–specific CD8+ T-cell clone was prepared. In brief, T cells from an HLA-A*0201+ donor were stained with HLA-A2/pp65495-503 tetramers, and subsequently single-cell sorted in a 96-well plate (Thermo) containing irradiated B-LCL feeder cells (1 × 105 cells/mL, irradiated with 70 Gy) and PBMCs from 3 healthy donors (1 × 106 cells/mL, irradiated with 30 Gy). One μg/mL leucoagglutinin PHA-L (Sigma-Aldrich) and 120 U/mL of recombinant IL-2 (Immunotools) were added. T-cell clones specific to pp65495-503 were selected using tetramer staining. Positive clones were restimulated and expanded during several stimulation cycles and frozen in aliquots that were freshly thawed before each use in an assay.
HLA-A2/pp65495-503 tetramer generation
HLA heavy chains and β2-microglobulin were constructed in pET plasmids and expressed in BL21 Escherichia coli strains. Heavy chain, β2-microglobulin and peptides were refolded by dialysis. HLA-tetramers with specificity for the CMV-derived peptide NLVPMVATV were complexed with HLA-A2 protein. Monomeric complexes were concentrated, biotinylated, HPLC purified on a BioSep SEC-S 3000 column (Phenomenex) and bound to either APC or PE-streptavidin (Sigma-Aldrich). Tetrameric product was HPLC purified.
Cross-presentation assay
DCs were loaded with either soluble HCMV pp65 protein (Miltenyi Biotec, purity > 95%, low endotoxin; < 10 EU/mL) or pp65:Ab IC (Abbiotec rabbit polyclonal anti-pp65) and incubated overnight at 37°C and 5% CO2 for processing. Blocking of FcRs was done by adding 10 μg/mL human IgG-Fc fragments (ITK), or 1 μg/mL FLIPr-like (kind gift from J.A. van Strijp and K.P. van Kessel). When endosomal or proteasomal antigen processing was assessed, DCs were incubated in the presence of 50mM MG132, 100μM lactacystin, 10μM epoxomicin (Cayman Chemical), 50μM chloroquine, 15μM leupeptin,or 50μM primaquine (all from Sigma-Aldrich, except for epoxomycin). After incubation, DCs were washed and HCMV pp65-specific CD8+ T cells were cocultured with pp65–loaded DCs for 4 to 6 hours in the presence of Golgistop (1/1500; BD Bioscience). Cells were subsequently stained for surface markers and presence of intracellular IFN-γ and TNF, followed by flow cytometry–based analysis.
DC maturation assay
Primary BDCA-1+ and BDCA-3+ or day 4½ monocyte-derived DCs (MoDCs) were incubated O/N in the presence of medium, pp65 antibodies, pp65 (3 μg/mL), pp65-IC (3 μg/mL pp65 complexed with 4-fold increased molar amount rabbit polyclonal anti-pp65 IgG) or Poly(I:C) [30 μg/mL (Sigma-Aldrich) and LPS (100 ng/mL (Sigma-Aldrich)]. Cells were subsequently harvested and analyzed for costimulatory marker expression using flow cytometry.
Flow cytometry
For staining, cells were first washed twice in PBS containing 2% FCS (Invitrogen) and 0.1% sodium azide (NaN3, Sigma-Aldrich). Next, antigen nonspecific binding was prevented by prior incubation of cells with 10% mouse serum (Fitzgerald). Cells were next incubated with combinations of pacific blue, phycoerythrin (PE), fluorescein isothiocyanate (FITC), allophycocyanin (APC), and PE-Cy7–conjugated mouse anti–human Ab (CD1c, CD3, CD8, CD11c, CD14, CD16, CD19, CD20, CD32, CD40, CD64, CD80, CD83, CD86, CD107a, CD141, and HLA-DR). Where indicated, after surface staining, T cells were washed twice in PBS/2% FCS/0.1% NaN3) and fixed, permeabilized, and intracellularly stained using mAbs to IFN-γ and TNF. Cells were acquired on FACSCanto II and analyzed using FACS Diva Version 6.13 (BD Bioscience) or FlowJo Version 7.6.5 software. Data were analyzed using GraphPad Prism 5.
Antigen uptake assay
To analyze DC uptake capacity, we incubated primary BDCA-1+ and BDCA-3+ DCs or day 5 MoDCs for 10 minutes (pulse) with 6 μg/mL eGFP (BioVision) or eGFP-IC, subsequently washed and chased for 50 minutes (37°C/ 5% CO2, in X-vivo 15 medium). Immune complexes were made using 4-fold increased molar amount compared with eGFP of rabbit anti-eGFP polyclonal IgG (Pierce Biotechnology). Next, cells were washed in PBS/2% FCS/0.1% NaN3, and eGFP fluorescence was measured by flow cytometry. To determine the effect of proteasome and endosomal blockers used in our cross-presentation experiments on uptake, we incubated DCs with MG132, lactacystin, epoxomicin, chloroquine, leupeptin, or primaquine during both the pulse and chase period (concentrations of inhibitors were previously described). Data shown are MFI values of eGFP treated DCs corrected for background MFI (MFI of non-eGFP treated DCs).
Confocal microscopy
MoDCs were cultured for 5 days in Lab-Tek II chambered coverglass dishes (Thermo), as described in “Monocyte-derived DC culture.” We coated slides with alcian blue 8GX (Klinipath) before addition of monocytes, and added eGFP or eGFP-IC for 1 or 4 hours. MoDCs were washed (PBS), fixed (3.7% paraformaldehyde/PBS, 10 minutes), and washed (PBS, 3 times). Next, MoDCs were permeabilized by adding saponin solution (1% BSA, 0.5% saponin, Sigma-Aldrich; in PBS, 30 minutes) and blocked (10% whole donkey or goat serum/PBS, 30 minutes, RT). EEA-1 was detected using goat anti–EEA-1 polyclonal IgG (Santa Cruz Biotechnology, in PBS, 45 minutes, RT), washed, and stained with donkey-anti–goat–Dyl647 (Jackson, in PBS/0.1% Tween, 45 minutes, RT). LAMP-1 was detected using mouse-anti–LAMP-1 polyclonal IgG (Biolegend, in PBS, 45 minutes RT), washed and stained with goat-anti–mouse–Dyl647 (Biolegend in PBS/0.1% Tween, 60 minutes, RT). After washing, we added 8 μL/well of MOWIOL (Calbiochem) supplemented with 1 μg/mL DAPI (Sigma-Aldrich). Cells were coverslipped and analyzed at RT using a LSM 710 confocal microscope, and “Plan-Apochromat” 63 × 1.40 oil DIC M27 objective (Zeiss). Image analysis was performed using Zen2009 (Zeiss) and ImageJ software including JACoP.18
Real-time PCR
Primary human BDCA-1+ and BDCA-3+ DCs were acquired as previously described. After cell sorting, total RNA was isolated using tripure (Roche) according to the manufacturer's instructions. cDNA was synthesized from up to 1 μg of total RNA using the iScript cDNA synthesis kit (Biorad). Real-time PCR was performed as described19 using IQ SYBR Green PCR Supermix (Biorad) and the CFX96 Touch Real-Time PCR Detection System (Bio-Rad), according to the manufacturer's instructions. PCR assays were done in triplicate. Data were calculated as values relative to GAPDH and further analyzed using Graphpad Prism 5.
Results
Potentiation of viral antigen cross-presentation by FcγR targeting
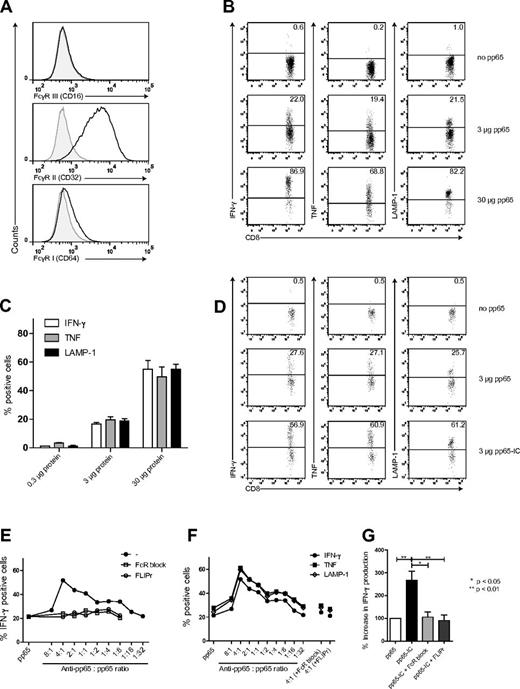
DC vaccination therapy to date uses MoDCs at large.3 We therefore first established our FcγR antigen targeting work in MoDCs, before moving into primary BDCA-3+ DCs. We proposed that FcγR antigen targeting may potentiate DC vaccination-induced CD8+ T-cell responses as in mice,15-17 and therefore assessed FcγR expression on MoDCs, cultured in the presence of GM-CSF and IL-4 for 5 days. FcγRII (CD32) was highly expressed, whereas expression of FcγRIII (CD16) and FcγRI (CD64) were low (Figure 1A, supplemental Figure 1A), confirming published data.19-21 MoDCs expressed the maturation markers CD40, CD80, CD83, and CD86 (supplemental Figure 1B), but could be further up-regulated after stimulation with LPS (100 ng/mL, O/N), classifying them as intermediately matured DCs. We thereby corroborate data shown in mice that intermediately matured DCs cross-present immune-complexed antigen.22
Human MoDCs express FcγRs that facilitate antigen cross-presentation. (A) FcγRI, II and III expression on human monocyte-derived DCs (MoDCs), cultured under serum-free conditions (n = 6). (B-C) Cross-presentation of pp65495-503 to CD8+ T cells (n = 6). (B) Representative plots of CD8+ T-cell activation. MoDCs were loaded with HCMV derived pp65 and cocultured with A2/NLVPMVATV specific T cells. Freshly thawed T cells were gated based on CD3 en CD8 expression and analyzed for activation-induced production of IFN-γ (left), TNF (middle), and LAMP-1 surface expression (right). (C) Summary (mean + SEM) of HCMV pp65495-503 cross-presentation. Bars represent production of IFN-γ (white), TNF (gray), and LAMP-1 (black) surface expression after coculture with MoDCs loaded with 0.3, 3, and 30 μg pp65 (n = 5-8). (D-G) Increased cross-presentation by FcγR targeting of pp65. (D) MoDCs were loaded with pp65 (top plots) or pp65-IC (bottom plots) and analyzed as in panel B. (E) HCMV pp65 was added across a range of Ab:Ag ratios and production of IFN-γ was analyzed (closed circles). Contribution of FcγR in IC-mediated cross-presentation by inclusion of FcγR-blocking reagents: purified IgG-Fc fragments (open squares) or recombinant S aureus–LIPr-like (open circles). (F) IFN-γ (closed circles), TNF (closed squares), and surface display of LAMP-1 (open diamonds) on CD8+ T cells. (G) Summary (mean + SEM) of IFN-γ production after IC-mediated cross-presentation in absence (black bar, n = 4) or presence of FcR blocking reagents (IgG-Fc-fragments, light gray; FLIPr-like, dark gray; n = 3).
Human MoDCs express FcγRs that facilitate antigen cross-presentation. (A) FcγRI, II and III expression on human monocyte-derived DCs (MoDCs), cultured under serum-free conditions (n = 6). (B-C) Cross-presentation of pp65495-503 to CD8+ T cells (n = 6). (B) Representative plots of CD8+ T-cell activation. MoDCs were loaded with HCMV derived pp65 and cocultured with A2/NLVPMVATV specific T cells. Freshly thawed T cells were gated based on CD3 en CD8 expression and analyzed for activation-induced production of IFN-γ (left), TNF (middle), and LAMP-1 surface expression (right). (C) Summary (mean + SEM) of HCMV pp65495-503 cross-presentation. Bars represent production of IFN-γ (white), TNF (gray), and LAMP-1 (black) surface expression after coculture with MoDCs loaded with 0.3, 3, and 30 μg pp65 (n = 5-8). (D-G) Increased cross-presentation by FcγR targeting of pp65. (D) MoDCs were loaded with pp65 (top plots) or pp65-IC (bottom plots) and analyzed as in panel B. (E) HCMV pp65 was added across a range of Ab:Ag ratios and production of IFN-γ was analyzed (closed circles). Contribution of FcγR in IC-mediated cross-presentation by inclusion of FcγR-blocking reagents: purified IgG-Fc fragments (open squares) or recombinant S aureus–LIPr-like (open circles). (F) IFN-γ (closed circles), TNF (closed squares), and surface display of LAMP-1 (open diamonds) on CD8+ T cells. (G) Summary (mean + SEM) of IFN-γ production after IC-mediated cross-presentation in absence (black bar, n = 4) or presence of FcR blocking reagents (IgG-Fc-fragments, light gray; FLIPr-like, dark gray; n = 3).
To test for cross-presentation ability, we cultured MoDCs in the presence of 0.3, 3, or 30 μg soluble HCMV pp65 protein or left MoDCs untreated (50 000 DCs/100 μL culture, 12-16 hours). We then added to DC cultures pp65495-503–specific CD8+ T cells recognizing HLA-A2/NLVPMVATV complexes (50 000 cells/well). T cells used were freshly thawed from frozen stock, which we had previously expanded from healthy donor blood T cells and characterized (supplemental Figure 1C-E). We measured T-cell stimulation (4-6 hours coculture), by induced cytokine production and LAMP-1 surface expression (Figure 1B-C). Background levels of IFN-γ–producing T cells were always between 0.1 and 2%. We observed the induced production of IFN-γ and TNF and surface displayed LAMP-1 in the majority of T cells: DC exposed to 0.3 μg pp65 protein induced IFN-γ at background levels, whereas 3 and 30 μg pp65 induced IFN-γ in 20% and 58% of T cells, respectively. TNF and LAMP-1 expression showed comparable results. Based on these data, we performed all of the following experiments using 3 μg pp65 protein. Next, we cultured MoDCs under serum-free conditions, and added pp65 across a range of Ab:Ag ratio's, allowing immune complex (IC) formation between pp65 and antigen-specific anti-pp65 IgG. We used polyclonal rabbit anti-pp65 because cross-reactivity with human FcγR is described.23 We confirmed optimal potentiation of cross-presentation when pp65 was administered in complex with 4-fold more molar amount of anti-pp65 IgG, as measured by production of IFN-γ in 52% of T cells. Again, TNF and surface display of LAMP-1 on CD8+ T cells showed comparable results. (Figure 1D-G, n = 4). Abs alone did not induce cytokine production (supplemental Figure 1G). The induced cross-presentation of anti-pp65:pp65 IC (hereafter referred to as pp65-IC) by MoDCs was mediated by FcγR targeting, as cross-presentation was completely blocked when FcγR blocking agents were included in the culture (FcγR block: inclusion of purified IgG Fc-fragments; FLIPr-like: Staphylococcus aureus derived formyl peptide receptor-like1 inhibitor protein (FLIPr-like; A. M. Stemerding, J. Köhl, A. Kuipers, J. Leusen, P. Boross, M. Nederend, A. Y. L. Weersink, J. G. J. van de Winkel, K. P. M. van Kessel, J. A. G. van Strijp, Identification of a Staphylococcal Fc gamma receptor inhibitory protein, manuscript submitted November 19, 2012). Of note, FcγR targeting of viral antigen also enhanced cross-presentation significantly in MoDCs cultured in serum-sufficient medium, that contains polyclonal IgG (supplemental Figure 1F). Taken together, the cross-presentation of viral soluble antigen by human MoDCs is potentiated by antigen targeting to FcγRs, at least in this culture system. Recent work in mice confirms that circulating antigen-specific IgG can potentiate systemic cross-presentation in mice.17
Increased cross-presentation of pp65-IC is not due to increased antigen uptake or maturation
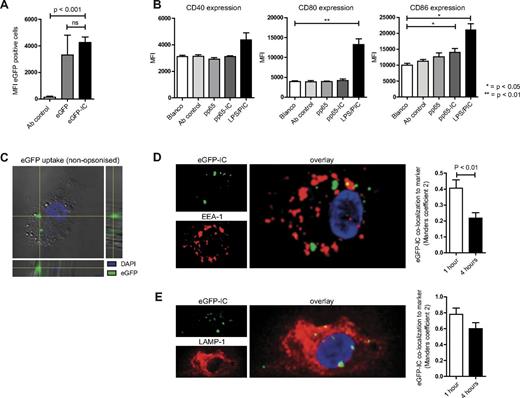
Increased cross-presentation could be explained by increased uptake of immune-complexed antigen, in analogy to mouse DCs in which IgG opsonization of E coli stimulates pathogen internalization.24 To determine whether ICs are better endocytosed compared with soluble antigen, we performed pulse-chase experiments using 5-day MoDCs and soluble eGFP protein. We compared uptake of eGFP (6 μg/well) with eGFP-IC (the same amount of eGFP precomplexed with 4-fold increased molar amount of anti-eGFP rabbit IgG) and determined eGFP uptake by DCs using flow-cytometry. We found comparable uptake of soluble eGFP and eGFP-IC by MoDCs (Figure 2A). We confirmed the presence of internalized soluble eGFP in 5-day MoDCs using confocal microscopy by Z-stack analysis (1 hour of MoDC culture in the presence of eGFP; Figure 2C).
Enhanced cross-presentation of immune-complexes is not due to increased antigen uptake or DC maturation. (A) Day 5 human MoDCs were cultured in the presence of eGFP (gray bar) or eGFP-IC (black bar) for 10 minutes (pulse), washed 3 times, and cultured for 1 hour (chase) to assess uptake efficiency (mean + SEM, n = 3). Data shown are MFI values corrected for background MFI (DCs cultured without eGFP). (B) Day 4½ human MoDCs were cultured overnight in the presence of medium (white bars), pp65 antibody (light gray bars), pp65 alone (gray bars), pp65-IC (dark gray bars), or 100 ng LPS and 30 μg poly(I:C) (PIC) to assess maturation status. Data shown are MFI values (mean + SEM, n = 3) of CD40 (left graph), CD80 (middle graph), and CD86 (right graph). (C-D) MoDCs were cultured on confocal slides and incubated with eGFP (C) or eGFP-IC (D). (C) Nonopsonized eGFP (green) is internalized by human MoDCs (bright field, nucleus visualized using DAPI). (D-E) MoDCs were allowed to internalize eGFP-IC for1 hour and were chased for 1 and 4 hours (37°C, analysis of 10-25 slides containing multiple DCs for each condition in 2 separate experiments). Cells were fixed and stained for confocal microscopy. Distribution of internalized eGFP-IC was quantified as percentage of vesicles positive for EEA-1 (D) or LAMP-1 (E).
Enhanced cross-presentation of immune-complexes is not due to increased antigen uptake or DC maturation. (A) Day 5 human MoDCs were cultured in the presence of eGFP (gray bar) or eGFP-IC (black bar) for 10 minutes (pulse), washed 3 times, and cultured for 1 hour (chase) to assess uptake efficiency (mean + SEM, n = 3). Data shown are MFI values corrected for background MFI (DCs cultured without eGFP). (B) Day 4½ human MoDCs were cultured overnight in the presence of medium (white bars), pp65 antibody (light gray bars), pp65 alone (gray bars), pp65-IC (dark gray bars), or 100 ng LPS and 30 μg poly(I:C) (PIC) to assess maturation status. Data shown are MFI values (mean + SEM, n = 3) of CD40 (left graph), CD80 (middle graph), and CD86 (right graph). (C-D) MoDCs were cultured on confocal slides and incubated with eGFP (C) or eGFP-IC (D). (C) Nonopsonized eGFP (green) is internalized by human MoDCs (bright field, nucleus visualized using DAPI). (D-E) MoDCs were allowed to internalize eGFP-IC for1 hour and were chased for 1 and 4 hours (37°C, analysis of 10-25 slides containing multiple DCs for each condition in 2 separate experiments). Cells were fixed and stained for confocal microscopy. Distribution of internalized eGFP-IC was quantified as percentage of vesicles positive for EEA-1 (D) or LAMP-1 (E).
Earlier work showed that efficient pp65 cross-presentation by DCs derived from HCMV-infected fibroblasts requires soluble factors secreted by the infected fibroblasts, causing their maturation.25 To investigate whether pp65-IC also induces DC maturation, we analyzed for induced up-regulation of maturation markers CD40, CD80 and CD86 (Figure 2B). To this end, we incubated MoDCs overnight with medium, anti-pp65, pp65, pp65-IC or a combination of LPS (100 ng/mL) and poly(I:C) (30 μg/mL). Of note, these experiments were performed without replating DCs to 96 wells plates, because the latter showed maturation independent of pp65 or toll-like receptor (TLR) stimulation, indicating mechanically induced maturation (T.W.H.F. and M.B., unpublished data, 2012).26,27 Incubation of pp65 or pp65-IC did not result in overt increase of the costimulatory markers CD40, CD80, or CD86, with only a minor increase in CD86 expression in pp65-IC treated MoDCs. Therefore, we concluded that increased cross-presentation of pp65-IC is not caused by increased costimulatory molecule expression. Finally, do endocytosed immune complexes localize to early and late endosomal compartments? To this end, we performed confocal microscopy analyses using MoDCs that were allowed to internalize eGFP-IC for1 hour and were chased for 1 and 4 hours (37°C). Although over time, eGFP-IC presence decreased in EEA1+ early endosomal compartments (P < .01, comparing eGFP fluorescence at 1 hour and 4 hours), eGFP fluorescence remained high in LAMP-1+ late endosomal compartments. Thus, immune complexes localize to early and late compartments of the endosomal pathway in human MoDCs.
Cross-presentation of FcγR-targeted viral antigen requires antigen processing in both the endosomal pathway and by the proteasome
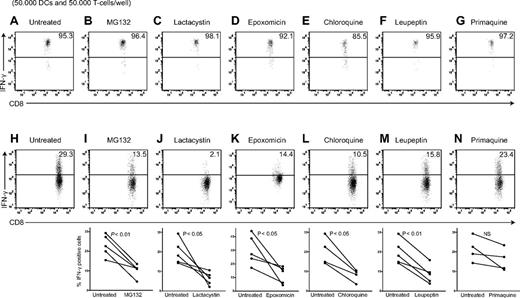
Increased antigen cross-presentation can also be attained by restraining the endosomal processing of IgG-coupled antigen, as was shown in mouse DCs.28 For immune complexes, the requirements for endosomal processing and proteasome-mediated peptide generation are not fully clear, particularly for human myeloid DCs.3,29 We therefore assessed in human MoDCs the role of antigen processing in the endosomal pathway and by the proteasome. We assessed cross-presentation of HCMV pp65 antigen to pp65–specific CD8+ T-cell clones, as reported,12,30 but in our case administered pp65 antigen as IgG-pp65 immune complexes to facilitate FcγR-mediated uptake. We treated human MoDCs with inhibitors of the proteasome (MG132, lactacystin, and epoxomicin), or with endosomal inhibitors that target either acidification (chloroquine), proteolysis (leupeptin), or surface-directed transport of recycling endosomes (primaquine). We first assessed the ability of MoDCs to stimulate pp65-specific CD8+ T-cell clones after inhibitor treatment, by addition of exogenous peptide. At inhibitor concentrations used, the MoDCs were still able to stimulate pp65-specific CD8+ T cells (Figure 3A-G, supplemental Figure 2A). We next confirmed that these inhibitors at concentrations used do not affect antigen uptake. We treated MoDCs with inhibitors by incubating eGFP-IC for 10 minutes (pulse) in the presence of inhibitors. DCs were then washed and incubated for 1 hour (chase), again in the presence of inhibitors, and analyzed for eGFP uptake by flow cytometry. Incubation of DCs in the presence of MG132, lactacystin, epoxomicin, chloroquine, leupeptin, or primaquine did not alter the capacity to internalize eGFP-IC (supplemental Figure 2B).
Cross-presentation of FcγR-targeted viral antigen requires antigen processing in both the endosomal pathway and by the proteasome. Human MoDCs were allowed to process 3 μg pp65-IC in absence (A-H) or presence of proteolysis inhibitors indicated (O/N, 37°C; B-G, I-N). To ascertain that inhibitors do not counteract HLA-A2–mediated presentation indiscriminately, DCs were loaded with 1−6 M NLVPMVATV peptide and T-cell activation assessed after 4 hours in the presence of Golgi-stop (A-G). Shown are representative plots of IFN-γ production by A2/NLVPMVATV-reactive T cells (n > 3 independent experiments, summarized in supplemental Figure 2) for peptide control experiments (A-G) and representative plots (top) and summarizing graphs for each inhibitor (H-N, n = 4-5). (B-I) MG132 proteasome inhibitor, 50μM. (C-J) Lactacysin proteasome inhibitor, 100μM. (D-K) Epoxomicin proteasome inhibitor, 10μM. (E-L) chloroquine endosomal acidification inhibitor, 50μM. (F-M) Leupeptin lysosomal cysteine protease inhibitor,15μM. (G-N) Primaquine recycling endosome inhibitor, 50μM.
Cross-presentation of FcγR-targeted viral antigen requires antigen processing in both the endosomal pathway and by the proteasome. Human MoDCs were allowed to process 3 μg pp65-IC in absence (A-H) or presence of proteolysis inhibitors indicated (O/N, 37°C; B-G, I-N). To ascertain that inhibitors do not counteract HLA-A2–mediated presentation indiscriminately, DCs were loaded with 1−6 M NLVPMVATV peptide and T-cell activation assessed after 4 hours in the presence of Golgi-stop (A-G). Shown are representative plots of IFN-γ production by A2/NLVPMVATV-reactive T cells (n > 3 independent experiments, summarized in supplemental Figure 2) for peptide control experiments (A-G) and representative plots (top) and summarizing graphs for each inhibitor (H-N, n = 4-5). (B-I) MG132 proteasome inhibitor, 50μM. (C-J) Lactacysin proteasome inhibitor, 100μM. (D-K) Epoxomicin proteasome inhibitor, 10μM. (E-L) chloroquine endosomal acidification inhibitor, 50μM. (F-M) Leupeptin lysosomal cysteine protease inhibitor,15μM. (G-N) Primaquine recycling endosome inhibitor, 50μM.
To test antigen-processing requirements, we performed cross-presentation experiments, now adding pp65-IC to DCs in the presence of relevant inhibitors (O/N). Pretreatment of MoDC with proteasome inhibitor MG132 (50μM), lactacysin (100μM), or epoxomicin (10μM) significantly reduced cross presentation of A2/NLVPMVATV complexes, as measured by IFNγ production by HCMV pp65495-503–specific CD8+ T cells (Figure 3H-K). We considered that endosomal antigen proteolysis may facilitate transfer of antigenic fragments across the endosomal membrane. Accordingly, proteasome digestion may perform final cleavage, to allow for generation of peptide cargo and assembly into peptide/HLA class I complexes. To clarify a possible role for endosomal antigen processing, we pretreated MoDCs with chloroquine (50μM) to inhibit endosomal acidification. Addition of chloroquine significantly reduced stimulation of pp65495-503–specific CD8+ T cells (Figure 3L). To confirm the need for endosomal proteolysis to cross-presentation in MoDCs, we pretreated MoDCs with the cysteine protease inhibitor leupeptin (15μM), which again resulted in diminished antigen stimulation of pp65495-503–specific CD8+ T cells (Figure 3M). Finally, inhibition of recycling endosome to cell surface transport using primaquine (50μM) did not diminish cross-presentation in this system (Figure 3N). These data together support that transition of antigen from endosome to cytosol is required for cross-presentation, while negating the possibility that all antigen processing occurs within endosomal constraints. Indeed, in human plasmacytoid DCs, cross-presentation of viral antigen is independent of proteasome digestion, with all processing being performed in the endosomal pathway.31 In ovalbumin cross-presentation by mouse DC systems, data collectively suggests that processing may occur in either the endosomal or cytosolic/proteasomal pathway, depending on the endocytic route taken (ie, choice of binding to endocytic receptors) and configuration of the antigen (ie, soluble or particulate).15,28,32,33 Our data in human MoDCs now shows that for HCMV pp65, FcγR-mediated uptake potentiates cross-presentation in a manner that requires processing both in the endosomal pathway and by the proteasome.
Human primary BDCA3+ DCs express FcγRs that contribute to antigen cross-presentation
Thus far, we have demonstrated that FcγR antigen targeting potentiates the cross-presentation of HCMV antigen by MoDCs. Are these findings applicable to human BDCA-3+ DCs? BDCA-3+ DCs are a recently described myeloid DC subset that exhibits superior cross-presentation abilities, but their possible application in trials commands preclinical analysis for favorable antigen targeting for yielding effector CD8+ T-cell activation. Affirmation that FcγR antigen targeting further potentiates cross-presentation efficiency by BDCA-3+ DCs should facilitate their application in DC vaccine clinical trials, as matched pairs of recombinant viral antigens and monoclonal Abs are available and can be generated in clinical grade quality.
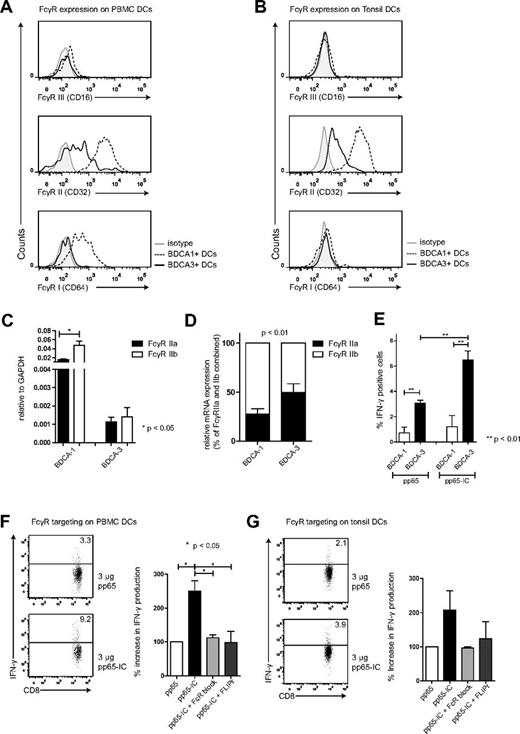
We therefore first assessed the presence of FcγRs on both BDCA-3+ and BDCA-1+ DC subsets that we extracted from human PBMCs and tonsils. Our flow cytometry gating strategy involved the selection for lineage negative, HLA-DR and CD11c-positive, BDCA-3 or BDCA-1–positive cells (supplemental Figure 3A) in human PBMCs (Figure 4A) and human tonsils (Figure 4B). In peripheral blood and tonsil DCs, both BDCA-3+ and BDCA-1+ expressed FcγRs. Similar to MoDCs, FcγRII (CD32) was expressed on BDCA-1+ and expressed at intermediate levels on BDCA-3+, whereas FcγRIII (CD16) expression was low on either subset. FcγRI (CD64) was expressed only on BDCA-1+ DCs (Figure 4A-B, supplemental Figure 3B). We then confirmed FcγRII expression by determining mRNA levels. FcγRII mRNA expression levels were again present in both DC subsets, and much higher in BDCA-1+ DCs compared with BDCA-3+ DCs (Figure 4C, RT-PCR amplification of FcγRII isoforms, relative to GAPDH). The relative expression of inhibitory FcγRIIb compared with stimulatory FcγRIIa appeared FcγRIIb-dominated in BDCA-1+ DCs, with comparable expression of stimulatory FcγRIIa and inhibitor FcγRIIb in BDCA-3+ DCs, possibly contributing to increased cross-presentation ability by BDCA-3+ DCs (Figure 4D, P < .01, n = 5).
FcγRs on human BDCA-3+ contribute to antigen cross-presentation. FcγRI, II and III expression on BDCA-1+ and BDCA-3+ DCs extracted from human blood (A) and tonsils (B; BDCA-1+, dashed line) and (BDCA-3+, solid line; n = 3). (C) FcγRIIa (black bars) and FcγRIIb (white bars) mRNA expression in BDCA-1+ and BDCA-3+ DCs extracted from human blood (mean + SEM, n = 5). (D) relative FcγRIIa (black bars) and FcγRIIb (white bars) mRNA expression in BDCA-1+ and BDCA-3+ DCs (mean + SEM, n = 5). (E) BDCA-1+ (white bar) and BDCA-3+ (black bar) were FACS-sorted (at least 98% purity) and cultured in the presence of 3 μg pp65 (left bars) or pp65-IC (right bars; O/N, 37°C). Next, A2/NLVPMVATVCD8+ T cells were added to DC cultures and cross-presentation was analyzed as in Figures 1 and 2 (mean + SEM, n = 3). (F-G) Representative plots and summary of IFN-γ production (mean + SEM) after IC-mediated cross-presentation in the absence (black bar) or presence of FcR blocking reagents (IgG-Fc fragments (light gray), recombinant S aureus–FLIPr-like (dark gray; n = 3 in panel F; n = 2 in panel G).
FcγRs on human BDCA-3+ contribute to antigen cross-presentation. FcγRI, II and III expression on BDCA-1+ and BDCA-3+ DCs extracted from human blood (A) and tonsils (B; BDCA-1+, dashed line) and (BDCA-3+, solid line; n = 3). (C) FcγRIIa (black bars) and FcγRIIb (white bars) mRNA expression in BDCA-1+ and BDCA-3+ DCs extracted from human blood (mean + SEM, n = 5). (D) relative FcγRIIa (black bars) and FcγRIIb (white bars) mRNA expression in BDCA-1+ and BDCA-3+ DCs (mean + SEM, n = 5). (E) BDCA-1+ (white bar) and BDCA-3+ (black bar) were FACS-sorted (at least 98% purity) and cultured in the presence of 3 μg pp65 (left bars) or pp65-IC (right bars; O/N, 37°C). Next, A2/NLVPMVATVCD8+ T cells were added to DC cultures and cross-presentation was analyzed as in Figures 1 and 2 (mean + SEM, n = 3). (F-G) Representative plots and summary of IFN-γ production (mean + SEM) after IC-mediated cross-presentation in the absence (black bar) or presence of FcR blocking reagents (IgG-Fc fragments (light gray), recombinant S aureus–FLIPr-like (dark gray; n = 3 in panel F; n = 2 in panel G).
Does FcγR antigen targeting potentiate the cross-presentation of HCMV pp65 by BDCA-3+ DCs? We administered either soluble HCMV pp65 protein or pp65-IC to BDCA-3+ and comparison with BDCA-1+ DCs (O/N), washed DCs, and added HCMV pp65–specific CD8+ T cells for coculture. We confirmed that cross-presentation was superior in BDCA-3+ DCs compared with BDCA-1+ DC (Figure 4E).11 This was not caused by differences in MHC class I expression (supplemental Figure 3C), neither did anti-pp65 antibody alone cause IFNγ secretion by pp65495-503–specific CD8+ T cells (supplemental Figure 3D). Similar as in Figure 1, HCMV antigen cross-presentation experiments were performed using serum-free medium, to avoid interference with serum-derived IgG. BDCA-3+ primary DCs extracted from PBMCs and tonsils induced 2- to 3-fold increased stimulation of pp65–specific T cells as measured by induced IFNγ production (Figure 4F-G). Also in primary BDCA-3+ DCs, pp65-IC–facilitated cross-presentation was fully mediated by FcγR, as pp65 T-cell stimulation was blocked using human IgG Fc fragments and recombinant FLIPr-like (Figure 4F-G). In a recent paper, lymphoid organ-resident DCs were able to cross-present MelanA long peptide antigen without prior in vitro activation, whereas blood DCs fail to do so.34 Others had shown earlier that human blood DCs do not cross-present antigen unless previously activated via TLR ligation.10,35 We here focused on the ability of BDCA-3+ DCs to cross-present immune complexed pp65 antigen, and show that both lymphoid organ and blood-derived BDCA-3+ DCs cross-presented pp65495-503/HLA-A2 complexes without prior stimulation.
Differential antigen uptake does not explain increased cross-presentation by BDCA3+ DCs
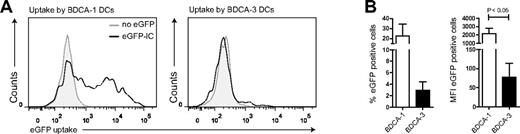
It is unclear whether human DC subtypes differ in their capacity to take up soluble proteins. To solve this question, we sorted BDCA-1+ and BDCA-3+ DCs and examined their ability to internalize eGFP immune complexes. Cells were incubated for 10 minutes with eGFP-IC, washed, and incubated for 1 additional hour (37°C). Unexpectedly, BDCA-1+ DCs endocytosed at least 10-fold more eGFP-IC protein compared with BDCA-3+ DCs (Figure 5A, representative histograms; 5B, (n = 3). Taken into account that BDCA-3+ DCs exhibit decreased efficiency in IC uptake, yet stimulate pp65495-503/HLA-A2–specific CD8+ T cells more efficiently than BDCA-1+ DCs, these results support the observation that BDCA-3+ DCs are superior in their antigen cross-presentation capacity.
BDCA-1+ rather than BDCA-3+ DCs internalize immune-complexed antigen. Human BDCA-1+ and BDCA-3+ DCs were cultured in the presence of eGFP-IC for 10 minutes, washed 3 times, and incubated for 1 hour (37°C). Uptake was analyzed using flow cytometry (A-B). Shown are representative plots of eGFP-IC (black line) uptake (A) and mean uptake (percentage positive cells, left or MFI, right) of BDCA-1+ (white bars) and BDCA-3+ DCs (black bars).
BDCA-1+ rather than BDCA-3+ DCs internalize immune-complexed antigen. Human BDCA-1+ and BDCA-3+ DCs were cultured in the presence of eGFP-IC for 10 minutes, washed 3 times, and incubated for 1 hour (37°C). Uptake was analyzed using flow cytometry (A-B). Shown are representative plots of eGFP-IC (black line) uptake (A) and mean uptake (percentage positive cells, left or MFI, right) of BDCA-1+ (white bars) and BDCA-3+ DCs (black bars).
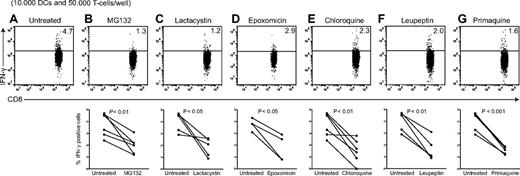
Various processing routes are shown to be relevant in cross-presentation biology. Involvement of these pathways seems distinctive between species, cell types, antigen, and route of antigen-uptake.3 Evidence for cytosolic entry of (partly) processed protein, and subsequent proteasome involvement is abundant,33,36-38 but proteasome-independent routes have been described as well.31,32 In case of proteasome-dependent cross-presentation, newly formed peptides could enter the ER39 or possibly be transported back into early endosomes,33,40 late endosomes or endo/lysosomes.41-43 These phagosomes contain MHCI loading complex components.36,37,40,44,45 To clarify mechanisms involved in BDCA-3+ DC cross-presentation, we tested the involvement of endosomal and proteasomal processing, analogous to experiments described in Figure 2. We cultured freshly isolated BDCA-3+ DCs in the presence of inhibitors (O/N) and confirmed antigen presentation ability by adding peptide after O/N treatment after we washed cells, followed by coculture with pp65-specific CD8+ T cells (supplemental Figure 4A). Treatment with pp65 or pp65-IC alone did not stimulate BDCA-3+ DC maturation, as levels of CD40, CD80, and CD86 were not increased compared with untreated or anti-pp65 antibody-treated BDCA-3+ DCs (16 hours of stimulation, positive control LPS/poly(I:C) supplemental Figure 4B). Cross-presentation in BDCA-3+ DCs required proteasome activity, as presentation of A2/NLVPMVATVcomplexes to HCMV pp65495-503–specific CD8+ T cells was diminished after proteasome inhibitor MG132 (50μM), lactacysin (100μM) or epoxomycin (10μM) treatment (Figures 6A-C). However, endosomal processing was also required, because addition of chloroquine (50μM) and leupeptin (15μM) resulted in significantly reduced stimulation of pp65495-503–specific CD8+ T cells (Figure 6D-E). Finally, inhibition of recycling of MHC class I molecules to the plasma membrane using primaquine (50μM) resulted in diminished cross-presentation, suggesting re-entry of peptides into the endosomes and subsequent loading onto recycling MHC-class I molecules. Taken together, FcγR targeting enhances the cross-presentation capability of BDCA-3+ DCs, that are already known as superior cross-presenting DCs. Mechanistically, we have clarified some of the intracellular pathways that support this exquisite cross-presentation capability. Our study supports the exploration of BDCA-3+ DCs for human vaccination strategies, in particular aimed at prevention of viral reactivation complications seen in immune compromised individuals.
Cross-presentation of FcγR-targeted antigen by human BDCA-3+ DCs requires antigen processing in both the endosomal pathway and by the proteasome. Human BDCA-3+ DCs were allowed to process 3 μg pp65-IC in the absence (A) or presence of proteolysis inhibitors indicated (O/N, 37°C; B-G). Next, DCs were washed and cocultured with A2/NLVPMVATVCD8+ T cells (4 hours in the presence of Golgi-stop), and IFN-γ production by T cells was measured as a read-out for cross-presentation. Shown are representative plots (top) and summarizing graphs for each inhibitor (n = 4-5). (B) MG132 proteasome inhibitor, 50μM. (C) Lactacysin proteasome inhibitor, 100μM. (D) Epoxomicin proteasome inhibitor, 10μM. (E) chloroquine endosomal acidification inhibitor, 50μM. (F) Leupeptin lysosomal cysteine protease inhibitor, 15μM. (G) Primaquine recycling endosome inhibitor, 50μM.
Cross-presentation of FcγR-targeted antigen by human BDCA-3+ DCs requires antigen processing in both the endosomal pathway and by the proteasome. Human BDCA-3+ DCs were allowed to process 3 μg pp65-IC in the absence (A) or presence of proteolysis inhibitors indicated (O/N, 37°C; B-G). Next, DCs were washed and cocultured with A2/NLVPMVATVCD8+ T cells (4 hours in the presence of Golgi-stop), and IFN-γ production by T cells was measured as a read-out for cross-presentation. Shown are representative plots (top) and summarizing graphs for each inhibitor (n = 4-5). (B) MG132 proteasome inhibitor, 50μM. (C) Lactacysin proteasome inhibitor, 100μM. (D) Epoxomicin proteasome inhibitor, 10μM. (E) chloroquine endosomal acidification inhibitor, 50μM. (F) Leupeptin lysosomal cysteine protease inhibitor, 15μM. (G) Primaquine recycling endosome inhibitor, 50μM.
Discussion
Most FcγR-mediated antigen uptake and cross-presentation studies were performed in mouse systems.15-17 It was our aim to show the possible applicability of such studies to human DC vaccination, in the context of viral reactivation after stem cell transplantation. In human DC trials, most DC vaccination therapies currently use MoDCs. We therefore started our studies in MoDCs. As antigen cross-presentation is crucial in antiviral responses, we extended our studies to BDCA-3+ DCs which were recently described as expert antigen cross-presenting DCs. Our second objective was therefore to test whether BDCA-3+ DC application in human DC vaccination therapy may be enforced by FcγR-targeted antigen loading. Our data collectively show that FcγR antigen targeting enhances the cross-presentation of immune-complexed HCMV-derived antigen that we used as a model viral antigen. Our work extends recent work in mice, that circulating antigen-specific IgG can potentiate systemic cross-presentation in mice.17
Mouse-based work suggests that in general, cross-presentation may involve NADPH-oxidase NOX2-mediated reduction of endosomal proteolysis,46 and the fusion of endoplasmic reticulum (ER) vesicles to endosomal counterparts by SNARE Sec22b.40 Clarification of cross-presentation mechanisms within human DCs might reveal how to improve their effective applicability toward T-cell stimulation in the clinic. We considered that DC vaccination as a treatment would be helped by optimizing the potency of DC subsets at stimulating antigen-specific CD8+ T-cell responses. We here show for MoDCs and BDCA-3+ DCs that processing by the proteasome, located in the cytosol, is required for cross-presentation, thus negating the possibility that all antigen processing occurs within endosomal constraints. In contrast, in human plasmacytoid DCs, cross-presentation of viral antigen is independent of proteasome digestion, with all processing being performed in the endosomal pathway.31 In ovalbumin cross-presentation by mouse DC systems, data collectively suggests that processing may occur in either the endosomal or cytosolic/proteasomal pathway, depending on the endocytic route taken (ie, choice of binding to endocytic receptors) and configuration of the antigen (ie, soluble or particulate).4,15,28,32,33 Our data in human MoDCs now shows that for HCMV pp65, FcγR-mediated uptake potentiates cross-presentation in a manner that requires processing both in the endosomal pathway and by the proteasome. Earlier flow cytometry–based work suggested that FcγR are not expressed on BDCA-3+ DCs found in PBMCs.47,48 Our work using inhibitors to FcγR-mediated antigen binding and signaling, however, show their functional relevance to cross-presentation. We corroborate our FcγR expression data by RT-PCR and by isolation of BDCA-3+ DCs from both human tonsils and PBMCs.
Our study has some limitations. Immune complexes did not stimulate antigen cross-presentation at all ratios of antibody:antigen. The optimal ratio we found for anti-pp65 IgG:pp65 was 4-fold molar excess compared with pp65 protein. For application in human DC vaccination, it will therefore be necessary to test individual antibody: antigen ratios for each pair used. Using the experimental setup we show in Figure 1E and F, such an endeavor should be relatively uncomplicated. Further, we showed that both antigen processing in the endosomal pathway and by the proteasome is instrumental to FcγR-mediated antigen cross-presentation. Additional enhancement of the CD8+ T-cell stimulatory activity by particularly BDCA-3+ DCs could be reached by modulation of the endosomal pathway, restraining superfluous antigen degradation. Such manipulations we considered may be less applicable to translation into the clinical setting, and therefore fell outside the scope of our current study. Finally, our BDCA-3+ DCs work was performed using primary DCs extracted from blood and tonsils. For application into clinical settings of these DCs, it will be necessary to optimize the culture conditions of these DCs from stem cell precursors, to generate a standardized and consistent supply of these cells for vaccination purposes.
Taken together, FcγR targeting enhances the cross-presentation capability of BDCA-3+ DCs, that are already known as superior cross-presenting DCs. Mechanistically, we have clarified some of the intracellular pathways that support this exquisite cross-presentation capability. Our study supports the exploration of BDCA-3+ DCs for human vaccination strategies, in particular those aimed at prevention of viral reactivation complications seen in immune-compromised individuals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the Boes laboratory and Dr M. Ressing and Dr S. Nierkens (both at the University Medical Center, Utrecht) for helpful discussions. The authors thank A. Stoppelenburg for help with qPCR experiments. The authors also thank Dr J. A. Van Strijp and Dr K. P. Van Kessel for providing FLIPr-like recombinant protein. The authors thank T. van den Broek and members of the ENT department for help in obtaining surplus human tonsil samples from anonymous donors.
This work was supported by a grant from The Netherlands Genomics Initiative (NGI)/Netherlands Organization for Scientific Research (NWO) to M.B., and by a KiKa grant to J.B.
Authorship
Contribution: T.W.H.F., E.B.C., J.J.B., and M.B. conceived the project and designed the experiments; T.W.H.F., E.B.C., M.K., and F.J.A. performed experiments; D.K. and D.B. provided essential reagents; T.W.H.F. and M.B. wrote the paper; and all authors commented on and approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marianne Boes, Department of Pediatric Immunology, University Medical Center Utrecht/Wilhelmina Children's Hospital, 3584 EA Utrecht, The Netherlands; e-mail: mboes@umcutrecht.nl.